��Ŀ����
����Ŀ����1�����и����У�
�ٽ��ʯ ��12C ��Һ�� �� 14C ��CH3CH2CH2CH3 ����ˮ
�� 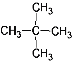 ��
��![]() ��C60��
��C60��
����ͬ�����������__________������Żش�����ͬ��������ͬ���칹�����________������ͬλ�ص���______________��
��2����a��MgBr2��b�����ʯ��c��NaOH��d���ɱ��������ʣ�������Ҫ��ش�����ĸ��Żش�����
���ۻ�ʱ����Ҫ�ƻ���ѧ������________���ۻ�ʱ��Ҫ�ƻ����ۼ�����_________���۵���ߵ���_____________��
��ֻ�����Ӽ���������___________���������Ӽ����й��ۼ���������_________�������Է��Ӽ���������ϵ���______________��
��3����֪0.2mol������������������ȫȼ�գ��������CO2��������Ϊ17.92L������£�����������ķ���ʽΪ_________________��д���ȸ�����1��̼��ͬϵ�������ӽṹ�к���3������-CH3���Ľṹ��ʽ______________________________��
���𰸡� �٢� �ߢ� �ڢ� d b b a c d C4H10 ![]()
��������
��1����ͬһ��Ԫ���γɵIJ�ͬ���ʻ�Ϊͬ�������壬������ͬ����������ǽ��ʯ��C60����ѡ�٢����ʽ��ͬ�ṹ��ͬ�Ļ����ﻥΪͬ���칹�壬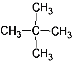 ��
��![]() �ķ���ʽ����C5H12���ṹ��ͬ������ͬ���칹����Ǣߢࣻ��������ͬ��������ͬ��ͬһ��Ԫ�صIJ�ͬ���ػ�Ϊͬλ�أ�12C��14C����ͬλ�أ���ѡ�ڢܡ�
�ķ���ʽ����C5H12���ṹ��ͬ������ͬ���칹����Ǣߢࣻ��������ͬ��������ͬ��ͬһ��Ԫ�صIJ�ͬ���ػ�Ϊͬλ�أ�12C��14C����ͬλ�أ���ѡ�ڢܡ�
��2�����廯þ�����������ۻ�ʱ����������ӣ����Ӽ����ƻ������ʯ�ۻ��ƻ����ۼ����ɱ��Ƕ�����̼���ۻ�ʱ���Ӽ����������ƻ������ۻ�ʱ����Ҫ�ƻ���ѧ�����Ǹɱ�����ѡd���ۻ�ʱ��Ҫ�ƻ����ۼ����ǽ��ʯ����ѡb�����ʯ��ԭ�Ӿ��壬�۵���ߵ��ǽ��ʯ����ѡb��
���廯þ��ֻ�����Ӽ����������Ӽ����й��ۼ����������������ƣ������Է��Ӽ���������ϵ��Ǹɱ���
��3����֪0.2mol������������������ȫȼ�գ��������CO2��������Ϊ17.92L������£���������̼�����ʵ�����17.92L��22.4L/mol��0.8mol���������������̼ԭ�Ӹ�����0.8mol��0.2mol��4�����Է���ʽΪC4H10���ȸ�����1��̼��ͬϵ�������飬����ӽṹ�к���3������-CH3�����������飬�ṹ��ʽΪ![]() ��
��

����Ŀ����ʯī�缫������е��ʵ��
ʵ��һ | ʵ��� | |
װ�� |
|
|
���� | a��d����ֽ����;b�����,�ֲ���ɫ;c�������Ա仯 | ����ʯī�缫���������ݲ���;n�������ݲ������� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ�������ǣ� ��
A. a��d����2H2O+2e-=H2��+2OH- B. b����2Cl--2e-=Cl2��
C. c�������˷�Ӧ��Fe-2e-=Fe2+ D. ����ʵ��һ��ԭ��,ʵ�����m��������ͭ




