��Ŀ����
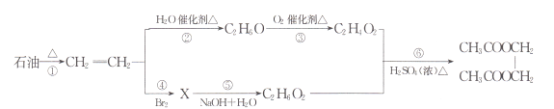
����Ŀ��ʯ����һ����Ҫ����Դ��ͨ��ʯ�ͷ���ɵõ����ͣ�������Ϊԭ�ϣ��ɺϳ�X��CH3COOCH=CH2������A�����ϩ����B�ܷ���������Ӧ��D����Է���������AС2����ش������й����⣺

(1)A�еĹ�����������___________________
(2)B��C�Ļ�ѧ����ʽ_____________________________
(3)��֪C��D�����ӳɷ�Ӧ����X���仯ѧ����ʽΪ________________________
(4)����˵����ȷ����_____
A���˴��ġ�ij���ա�ָ���Ƿ��� B��B��һ���������������������ӳɷ�Ӧ
C��X������ˮ����ʹ���Ը��������Һ��ɫ D������������Ȼ�̼��Һ����A��D
(5)F��X��ͬ���칹�壬1molF����4molAg(NH3)2OH��Ӧ��д��F���ܵĽṹ��ʽ_____________
���𰸡�̼̼˫�� 2CH3CHO+O2![]() 2CH3COOH CH��CH+CH3COOH
2CH3COOH CH��CH+CH3COOH![]() CH3COOCH=CH2 ABC OHCCH2CH2CHO��CH3CH(CHO)2
CH3COOCH=CH2 ABC OHCCH2CH2CHO��CH3CH(CHO)2
��������
A�����ϩ����AΪ��ϩ����ϩ����������ȩ����ȩ������������ ���BΪ��ȩ��CΪ���D����Է���������AС2��Ϊ26��DΪ��Ȳ���������Ȳ�����ӳɷ�Ӧ����X(CH3COOCH=CH2)���ݴ˷������
(1)AΪ��ϩ�����й�����Ϊ̼̼˫�����ʴ�Ϊ��̼̼˫����
(2)B��CΪ��ȩ�����������ᣬ��Ӧ�Ļ�ѧ����ʽΪ2CH3CHO+O2![]() 2CH3COOH���ʴ�Ϊ��2CH3CHO+O2
2CH3COOH���ʴ�Ϊ��2CH3CHO+O2![]() 2CH3COOH��
2CH3COOH��
(3)C��D�����ӳɷ�Ӧ����X����Ӧ�Ļ�ѧ����ʽΪCH��CH+CH3COOH![]() CH3COOCH=CH2���ʴ�Ϊ��CH��CH+CH3COOH
CH3COOCH=CH2���ʴ�Ϊ��CH��CH+CH3COOH![]() CH3COOCH=CH2��
CH3COOCH=CH2��
(4)A�����ͷ����е�̼���ϳ���������ij�������õ���̼���϶̵ķ��ӣ��ù����ѻ����ѽ⣬��A��ȷ��B��BΪ��ȩ����һ���������������������ӳɷ�Ӧ�����Ҵ�����B��ȷ��
C��X(CH3COOCH=CH2)Ϊ���࣬������ˮ������̼̼˫������ʹ���Ը��������Һ��ɫ����C��ȷ��D����ϩ����Ȳ����ʹ������Ȼ�̼��Һ��ɫ�����ܼ��𣬹�D������ȷ����ABC���ʴ�Ϊ��ABC��
(5)F��X(CH3COOCH=CH2)��ͬ���칹�壬1molF����4molAg(NH3)2OH��Ӧ��˵��F�к���2��ȩ����F���ܵĽṹ��ʽ��OHCCH2CH2CHO��CH3CH(CHO)2���ʴ�Ϊ��OHCCH2CH2CHO��CH3CH(CHO)2��