��Ŀ����
��15�֣��ں� b mol AlCl3����Һ�м��뺬 a mol NaOH����Һ��
��1������a��3b�������ι�����ʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��2������a��4bʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��3������3��a/b��4ʱ�����г������ɣ������в��ֳ����ܽ⣬��ʱAl��OH��3�����ʵ���Ϊ mol��
��4������д��AlCl3��NaOH��Һ��Ӧʱ�����йص����ӷ���ʽ��ѧ����ʽ��
�٣�
�ڣ�
�ۣ�
��1������a��3b�������ι�����ʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��2������a��4bʱ������Al��OH��3 ���������ʵ���Ϊ mol��
��3������3��a/b��4ʱ�����г������ɣ������в��ֳ����ܽ⣬��ʱAl��OH��3�����ʵ���Ϊ mol��
��4������д��AlCl3��NaOH��Һ��Ӧʱ�����йص����ӷ���ʽ��ѧ����ʽ��
�٣�
�ڣ�
�ۣ�

��

��ϰ��ϵ�д�
�����Ŀ

 Һ
Һ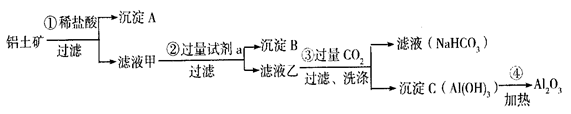
 A��B�ijɷֱַ��� �� ������� �е��Լ�a�� ��
A��B�ijɷֱַ��� �� ������� �е��Լ�a�� �� �д���Fe3���IJ���������__________ _��
�д���Fe3���IJ���������__________ _�� �������ȼ��ڸ����·����ķ�Ӧ�����ȷ�Ӧ���磺2Al��
�������ȼ��ڸ����·����ķ�Ӧ�����ȷ�Ӧ���磺2Al�� ����Ϣ�ش��������⣺
����Ϣ�ش��������⣺ ��6��θ��ƽ������θ�ᣨHCl������ij���ҩ����к��е���Ч�ɷ�������������������ԭ���ǣ������ӷ���ʽ��ʾ���������������������������������� ��
��6��θ��ƽ������θ�ᣨHCl������ij���ҩ����к��е���Ч�ɷ�������������������ԭ���ǣ������ӷ���ʽ��ʾ���������������������������������� �� ��þ���������������ɺϽ�þ���Ͻ��ǿ�ȸߣ���е���ܺá��С���������������������Ȼ���е�þԪ����Ҫ�����ں�ˮ�С���ˮ��þ���ܴ���ԼΪ1.8��1015 t���Ӻ�ˮ�У���Ҫ����Na+��Cl����Mg2+�����ӣ���ȡþ�Ĺ�������ͼ���£�
��þ���������������ɺϽ�þ���Ͻ��ǿ�ȸߣ���е���ܺá��С���������������������Ȼ���е�þԪ����Ҫ�����ں�ˮ�С���ˮ��þ���ܴ���ԼΪ1.8��1015 t���Ӻ�ˮ�У���Ҫ����Na+��Cl����Mg2+�����ӣ���ȡþ�Ĺ�������ͼ���£� �����ռ���CO2��Ȼ����10mLŨNaO
�����ռ���CO2��Ȼ����10mLŨNaO H��Һ��Ѹ���ܷ������ڣ����Թ۲쵽������ͻȻ����ˣ�ԭ���� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ�����ӷ���ʽΪ �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
H��Һ��Ѹ���ܷ������ڣ����Թ۲쵽������ͻȻ����ˣ�ԭ���� �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ�����ӷ���ʽΪ �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�