��Ŀ����
����Ŀ����A������ͼ�У��ʺɱ�������ֵΪ42��̼����Ԫ�ص�������Ϊ6:1����˴Ź��������������壬��������Ϊ1:2:3��A�������л���֮��Ĺ�ϵ���£�

��֪��CH2��CH2![]() HOCH2CH2OH���ش��������⣺
HOCH2CH2OH���ش��������⣺
(1)�л���B�ķ���ʽ___________________________��
(2)�߾���F�ṹ��ʽΪ___________________��
(3)д��C�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽ___________________________��
(4)E��һ�������¿������Ӧ����һ����Ԫ���л���H��H�Ľṹ��ʽ________.��
(5)д������G�Ļ�ѧ����ʽ_____________________________________________��
���𰸡� ��C3H8O2 ![]()
![]()
 n
n![]()
![]()
![]() +��n-1��H2O
+��n-1��H2O
��������������������⿼���л��ƶϣ��漰�л������ʽ�ͽṹ��ʽ��ȷ�����л������ʽ�ͽṹ��ʽ����д���л���Ӧ����ʽ����д��A������ͼ���ʺɱ�������ֵΪ42��A����Է�������Ϊ42����A��n��C����n��H��=![]() ��
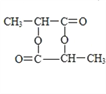
�� ![]() =1:2��A��ʵ��ʽΪCH2��A�ķ���ʽΪ��CH2��x��14x=42�����x=3��A�ķ���ʽΪC3H6��A�ĺ˴Ź����������������ҷ�������Ϊ1:2:3��A�Ľṹ��ʽΪCH2=CHCH3��A�����Ӿ۷�Ӧ���ɵĸ߾���F�Ľṹ��ʽΪ
=1:2��A��ʵ��ʽΪCH2��A�ķ���ʽΪ��CH2��x��14x=42�����x=3��A�ķ���ʽΪC3H6��A�ĺ˴Ź����������������ҷ�������Ϊ1:2:3��A�Ľṹ��ʽΪCH2=CHCH3��A�����Ӿ۷�Ӧ���ɵĸ߾���F�Ľṹ��ʽΪ![]() ��A��B���������֪�ķ�Ӧ��B�Ľṹ��ʽΪ
��A��B���������֪�ķ�Ӧ��B�Ľṹ��ʽΪ![]() ��B��C�������Ĵ�������C�Ľṹ��ʽΪ
��B��C�������Ĵ�������C�Ľṹ��ʽΪ![]() ��C��Cu��OH��2����ʱ��C��-CHO���������ữ��õ���D�Ľṹ��ʽΪ
��C��Cu��OH��2����ʱ��C��-CHO���������ữ��õ���D�Ľṹ��ʽΪ![]() ��D��H2�����ӳɷ�Ӧ����E��E�Ľṹ��ʽΪ
��D��H2�����ӳɷ�Ӧ����E��E�Ľṹ��ʽΪ![]() ��E�к��ǻ����Ȼ���E�������۷�Ӧ���ɸ߾���G��G�Ľṹ��ʽΪ
��E�к��ǻ����Ȼ���E�������۷�Ӧ���ɸ߾���G��G�Ľṹ��ʽΪ![]() ��
��
��1��B�Ľṹ��ʽΪ![]() ��B�ķ���ʽΪC3H8O2��
��B�ķ���ʽΪC3H8O2��
��2���߾���F�Ľṹ��ʽΪ![]() ��
��
��3��C�Ľṹ��ʽΪ![]() ��C������Cu��OH��2��Ӧ�Ļ�ѧ����ʽΪ
��C������Cu��OH��2��Ӧ�Ļ�ѧ����ʽΪ![]() +2Cu��OH��2+NaOH
+2Cu��OH��2+NaOH![]()
![]() +Cu2O��+3H2O��
+Cu2O��+3H2O��
��4��E�Ľṹ��ʽΪ![]() ��2����Eͨ��������Ӧ�γ���Ԫ���л���H��H�Ľṹ��ʽΪ
��2����Eͨ��������Ӧ�γ���Ԫ���л���H��H�Ľṹ��ʽΪ ��
��
��5��G��E�������۷�Ӧ���ɣ�����G�Ļ�ѧ����ʽΪn![]()
![]()
![]() +��n-1��H2O��
+��n-1��H2O��
�����͡��ƶ���
��������
18
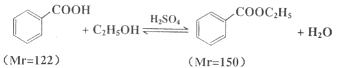
����Ŀ��������������C9H10O2������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��
��֪��

ʵ�鲽�����£�����100 mLԲ����ƿ�м���12.20 g�����ᡢ25mL�Ҵ�����������20mL�����飬�Լ�4mLŨ���ᣬ��Ͼ��Ȳ������ʯ��������ͼ��ʾװ�������������¶���65��70����Ȼ���2h����Ӧʱ������-�Ҵ�-ˮ���γɡ���������е�62.6�棩��������������÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���
�ڷ�Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
�۽���ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У���������Na2CO3����Һ�����ԡ�
���÷�Һ©���ֳ��л��㣬ˮ����25mL������ȡ��Һ��Ȼ��ϲ��л��㡣�����Ȼ��ƣ��Դֲ�Ʒ��������װ����ͼ��ʾ���������������Ѻ�������£�����210��213�����֡�
�ݼ���ϸ�ò�Ʒ���Ϊ12.86mL.
(1)�������ʹ�÷�ˮ�����Ϸ����ȥˮ��Ŀ����_________________��
(2)�������Ӧ������ֵ��¶���___________________��
A��65��70�� B��78��80�� C��85��90�� D��215��220��
(3)���������Na2CO3���벻�㣬�ڲ��������ʱ��������ƿ�пɼ����������ɣ������������ԭ����_____________��
(4)������з�Һ����������ȷ����__________��
A.ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ�����������Һ©����ת������ҡ
B.��ҡ���κ����Һ©���¿ڵIJ���������
C.��������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D.��Һ����ʱ����Һ©���е��²�Һ�����¿ڷų���Ȼ���ٽ��ϲ�Һ�����¿ڷų�
����װ��ͼ������A��������___________���ڲ�����м����Ȼ��Ƶ�������_________��
(5)��ʵ���Ʒ�IJ���Ϊ____________��
���𰸡� ������ƽ�ⲻ�����������ƶ�����߱������������� C �����������л��б����ᣬ��������100��ʱ�������� AB ������ƿ ��ˮ�� 90.02%
��������������������⿼�鱽�����������Ʊ���
��1����Ӧ![]() +CH3CH2OH
+CH3CH2OH![]()
![]() +H2OΪ���淴Ӧ��ʹ�÷�ˮ�����Ϸ����ȥˮ����С������Ũ�ȣ�������ƽ�ⲻ��������Ӧ�����ƶ�����߱����������IJ��ʡ�
+H2OΪ���淴Ӧ��ʹ�÷�ˮ�����Ϸ����ȥˮ����С������Ũ�ȣ�������ƽ�ⲻ��������Ӧ�����ƶ�����߱����������IJ��ʡ�
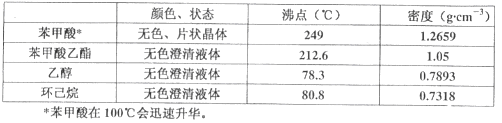
��2���������⣬��Ӧʱ������-�Ҵ�-ˮ���γ����������������������ƿ�ڵı����������л����Ҵ��������顢������������������������������������������е��Ҵ��������飬�Ҵ��ķе�Ϊ78.3����������ķе�Ϊ80.8���������������ķе�Ϊ212.6�������Բ�����Ӧ������ֵ��¶���85~90������ѡC��
��3���������м���Na2CO3��ȥ�����������л��еı���������ᣬ��Na2CO3���벻�㣬������û����ȫ��ȥ������������ʱ������ƿ�пɼ����̵�ԭ���ǣ������������л��б���������������������100��ʱ����������
��4��A��Ϊ��ʹ���Ѻ�ˮ��Һ��ֽӴ���ˮ��Һ�м�������ת������Һ©���к������ϲ�����������Һ©����ת������ҡ��A����ȷ��B��Ϊ��ֹ��Һ©������ѹ����������������ҡ���κ����Һ©���¿ڵIJ�����������B����ȷ��C����������ҡ���������轫��Һ©����������̨�Ͼ��á���Һ��ֲ㣬C�����D����Һ����ʱ����Һ©���е��²�Һ�����¿ڷų���Ȼ���ϲ�Һ����Ͽ��㵹������D�����ѡAB������װ��ͼ������A��������������ƿ���ڲ������м���CaCl2����������Ϊ��ˮ������ȥˮ��
��5�������Ҵ��������Ա���������������ɵı�����������![]() ~
~![]() ��n������������������=n�������ᣩ=
��n������������������=n�������ᣩ=![]() =0.1mol��m������������������=0.1mol
=0.1mol��m������������������=0.1mol![]() 150g/mol=15g����ʵ���Ʒ�IJ���=
150g/mol=15g����ʵ���Ʒ�IJ���=![]() 100%=90.02%��
100%=90.02%��

����Ŀ���״���һ����Ҫ���л�ԭ�ϣ��ڴ����������£�CO��H2��Ӧ�����ɼ״� (CH3OH) ������CH4����Ӧ������
��Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H1=-90.0kJ/mol
CH3OH(g) ��H1=-90.0kJ/mol
�� Ӧ��CO(g)+3H2(g)![]() CH4(g) + H2O(g) ��H2
CH4(g) + H2O(g) ��H2
��Ӧ�� CH4(g)+2H2O(g)![]() CO2(g)+ 4H2(g) ��H3=+125.0 kJ/mol
CO2(g)+ 4H2(g) ��H3=+125.0 kJ/mol
��Ӧ��CO(g)+ H2O(g)![]() CO2(g) + H2(g) ��H4=-25.0 kJ /mol
CO2(g) + H2(g) ��H4=-25.0 kJ /mol
K1��K2��K3��K4�ֱ��ʾ��Ӧ����������������ƽ�ⳣ����
�ش�����������
��1����Ӧ����ƽ�ⳣ���ı���ʽΪK2=______________��K2��K3��K4�Ĺ�ϵΪK2=______________����H2=____________kJ/mol��
��2��ͼ1������ȷ��ʾ��Ӧ����ƽ�ⳣ��(lgK1) ���¶ȱ仯������Ϊ______________����������ĸ�������ж�����Ϊ______________________________________________________________��

��3�����º��ݵ������£����������˵����Ӧ���ﵽƽ��״̬����__________________��
A.2v�� (H2)=v��(CH3OH) B.���������ܶȲ��ٸı�
C.��������ƽ��Ħ���������ٸı� D.��������ѹǿ���ٸı�
��4��Ϊ̽����ͬ������CO��H2����CH3OH��ѡ����Ч����ijʵ���ҿ���CO��H2�ij�ʼͶ�ϱ�Ϊ1��3����ʵ�飬�õ�����������
T/K | ʱ��/min | �������� | �״��ĺ���(%) |
450 | 10 | CuO-ZnO | 78 |
450 | 10 | CuO-ZnO-ZrO2 | 88 |
450 | 10 | ZnO-ZrO2 | 46 |

���ɱ�1��֪����Ӧ������Ѵ���Ϊ______________��ͼ2��a��b��c��d�ĵ��Ǹ��¶���COƽ��ת���ʵ���_________________________________��
�����������COת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��_________________��
A.ʹ�ô���CuO-ZnO-ZrO2 B.�ʵ����ͷ�Ӧ�¶�
C.����CO��H2�ij�ʼͶ�ϱ� D.�����£��ٳ���a molCO��3a mol H2
��5����֪1000������ӦCO(g)+ H2O(g)![]() CO2(g) + H2(g) K4=1.0�����¶��£���ijʱ����ϵ��CO��H2O��CO2��H2��Ũ�ȷֱ�Ϊ3molL-1��1molL-1��4molL-1��2molL-1�����ʱ������Ӧ��v��(CO)_______v��(CO) �����������������=�����ﵽƽ��ʱc(CO)=___________ molL-1��
CO2(g) + H2(g) K4=1.0�����¶��£���ijʱ����ϵ��CO��H2O��CO2��H2��Ũ�ȷֱ�Ϊ3molL-1��1molL-1��4molL-1��2molL-1�����ʱ������Ӧ��v��(CO)_______v��(CO) �����������������=�����ﵽƽ��ʱc(CO)=___________ molL-1��