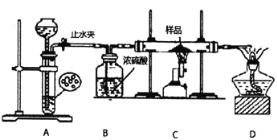
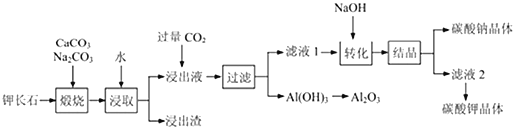
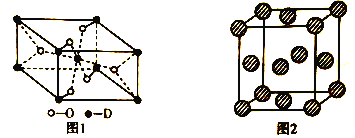
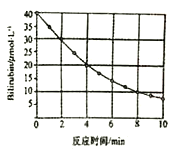
��Ŀ����
����Ŀ����ʹ������к͵ζ����ⶨ���۰״�������(g��100mL��1)��
��.ʵ�鲽��
��1������100ml����״���Һ:��(����������)��ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�(����������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00 mL����ƿ�У������еμ�2����ָʾ����![]()
��3����ȡʢװ0.1000 mol��L��1 NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���ΪmL��
��4���ζ�����ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��5����.ʵ���¼
�ζ�����ʵ������(mL) | 1 | 2 | 3 | 4 |
V(��Ʒ) | 20.00 | 20.00 | 20.00[ | 20.00 |
V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.���ݴ���������
�ٰ�ʵ���������ݣ��ɵ�c(���۰״�)��mol��L��1��
���۰״���������g��100 mL��1��
���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�������д���)��
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
���𰸡�
��1����ʽ�ζ��ܣ���10mL��Һ�ܣ�,100ml����ƿ
��2����̪
��3��0.60
��4����Һ����ɫǡ�ñ�Ϊ��ɫ(��ۺ�ɫ),���ڰ�����ڲ���ɫ
��5��0.75,4.5,ab
���������⣺��1������ʽ�ζ���(��10mL��Һ��)(��ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL ����ƿ�ж��ݣ�ҡ�ȼ��ô���״���Һ����2��ʳ����NaOH��Ӧ������ǿ�������Σ���Һ�ʼ��ԣ�Ӧѡ����Ա�ɫ��Χ�ڵ�ָʾ����̪����3���ζ���Һ��Ķ���0.60mL����4��NaOH�ζ�ʳ���յ�Ϊ����Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫ��
�ٵ�1�εζ�������Դ����쳣ֵ��Ӧ��ȥ��3�����ĵ�NaOH��Һ�����Ϊ��15.00mL��15.05mL��14.95mL����NaOH��Һ�������ƽ��ֵΪ15.00mL����10mL���۰״���Ʒ�� CH3COOH �����ʵ���Ũ��Ϊx����
CH3COOH�� | NaOH |
1mol | 1mol |
0.2x | 0.1000mol/L��0.015L |
��c(���۰״�)�� ![]() =0.75mol/L
=0.75mol/L
��Ʒ������Ϊ0.75mol/L��0.2L��60g/mol�� ![]() =4.50g/100mL����a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ����ҺŨ�Ƚ��ͣ����V(��)ƫ����c(����)=
=4.50g/100mL����a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ����ҺŨ�Ƚ��ͣ����V(��)ƫ����c(����)= ![]() ������֪c(����)ƫ��a��ȷ��b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V(��)ƫ����c(����)=
������֪c(����)ƫ��a��ȷ��b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V(��)ƫ����c(����)= ![]() ������֪c(����)ƫ��b��ȷ��c����ƿ�м������״���Һ���ټ�����ˮ����V(��)��Ӱ�죬����c(����)=
������֪c(����)ƫ��b��ȷ��c����ƿ�м������״���Һ���ټ�����ˮ����V(��)��Ӱ�죬����c(����)= ![]() ������֪c(����)���䣬c����d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������Һ���ʵ���ƫС�����V(��)ƫС������c(����)=
������֪c(����)���䣬c����d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������Һ���ʵ���ƫС�����V(��)ƫС������c(����)= ![]() ������֪c(����)ƫС��d����ѡab��
������֪c(����)ƫС��d����ѡab��
�����㾫����ͨ�������������к͵ζ��������к͵ζ�ʵ��ʱ��������ˮϴ���ĵζ������ñ�Һ��ϴ����װ��Һ�����ô���Һ��ϴ������ȡҺ�壻�ζ��ܶ���ʱ�ȵ�һ�����Ӻ��ٶ������۲���ƿ����Һ��ɫ�ĸı�ʱ���ȵȰ������ɫ�����Ϊ�ζ��յ㼴���Խ����⣮

 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�