��Ŀ����
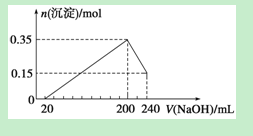
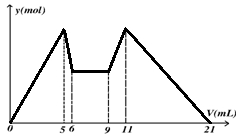
��ͼ����MgCl2��AlCl3�����Һ�У��Ⱥ�����Լ�A��Bʱ���ó������ʵ���y��mol�� ���Լ����V��mL)��Ĺ�ϵͼ����ʼ�μ�6mL�Լ�A������μ�A���ɣ�֮��ĵ��Լ�B�����½�����ȷ����
A��A������NaOH��B���������ᣬ��2 c(A)��c(B)
B��ԭ���Һ�У�c(Al3+)��c(Mg2+)��c(Cl��)��1��2��7
C��A������Ba(OH)2��B���������ᣬ��c(A)��2c(B)
D����A��B��ΪһԪǿ���һԪǿ���μ�7mL�Լ�A��ĵ��Լ�B

A��A������NaOH��B���������ᣬ��2 c(A)��c(B)
B��ԭ���Һ�У�c(Al3+)��c(Mg2+)��c(Cl��)��1��2��7
C��A������Ba(OH)2��B���������ᣬ��c(A)��2c(B)
D����A��B��ΪһԪǿ���һԪǿ���μ�7mL�Լ�A��ĵ��Լ�B
D
���������Aѡ����ݼ�����������ܽ��ϵͼ��֪���ȼ����aΪNaOH��֮������bΪ�ᣬ������5-6ml�����ܽ�ӦΪAl(OH)3+OH-=AlO2-+2H2O�����ʵ���֮��Ϊ1:1��9-11ml���ɳ���ΪAlO2-+H++H2O=Al(OH)3�����ʵ���֮��Ϊ1:1���ʶ��ߵ�Ũ�ȱ� c(A)��2c(B)����A����Bѡ��Ӽ���5 mL A���ɳ�����࣬�ټ�����1 mL A���������ٵ���Сֵ����Ӧ��Ӧ�����ӷ���ʽΪ��Al(OH)3+OH-=AlO2-+2H2O���ɴ˿�֪��n(Al3+)=n��Al(OH)3��=(1��6��10-3)mol����ǰ5 mL NaOH�γ�����������֪��2n(Mg2+)+3n��Al3+��=(5��10-3��6)mol������n(Mg2+)=(1��6��10-3) mol����Һ�������������������ȵã�n(Cl-)=(5��10-3��6)mol������Һ��c(Mg2+)��c(Al3+)��c(Cl-)=1��1��5����B����Cѡ���aΪBa(OH)2 ��bΪ���ᣬ��6-9ml��Ӧ���г������ı仯���������ޱ仯����C����Dѡ����ȷ����A��B��ΪһԪǿ���һԪǿ����ݶ��ߵ�Ũ�ȱ� c(A)��2c(B)����D��ȷ��

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
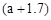 g������˵������ȷ����
g������˵������ȷ����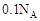
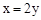
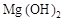 ��
��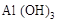 �Ļ����
�Ļ����