��Ŀ����
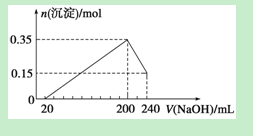
����Mg��Al��ɵ�һ�������Ļ����Ͷ��500 mL ϡ�����У�����ȫ���ܽⲢ�������塣����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ����ͼ��ʾ��������˵����ȷ����

| A��������Mg��Al��ɵĻ���������Ϊ8g |
| B����������ʵ���Ũ��Ϊ1 mol��L��1 |
| C�����ɵ�H2�ڱ�״���µ����Ϊ11.2L |
| D��NaOH��Һ�����ʵ���Ũ��Ϊ3.75 mol��L��1 |
B
���������A���������Ϊ0.15��24+0.35��27="9" g������B���������Ƶ����ʵ���Ũ����0.2mol��0.04L��5.0mol/L����������ֵ�������������ǣ�0.2L��5mol/L����2��0.5mol������ϡ�����Ũ����0.5mol��0.5L��1.0mol/L����ȷ��C��ϡ���ᷴӦ���ɵ�������0.3mol��0.15mol��0.45mol����״���µ������10.08L������D�����ݷ�ӦAl(OH)3��NaOH=NaAlO2��2H2O��֪���������Ƶ����ʵ���Ũ����0.2mol��0.04L��5.0mol/L������

��ϰ��ϵ�д�
�����Ŀ
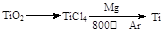
 MgO+H2�� Mg3N2 +6H2O =3Mg(OH)2+2NH3��
MgO+H2�� Mg3N2 +6H2O =3Mg(OH)2+2NH3��
