��Ŀ����
A��B��C��DΪ���ֶ���������Ԫ�أ�����A��Cͬ�壬B��Cͬ���ڣ�D��A��B����ͬ���ڣ�Aԭ�������������Ǻ�����Ӳ�����3����B���������������۴�����Ϊ6��(1)B��ԭ�ӽṹʾ��ͼ��__________________��ʵ�����Ʊ�B���ʵ����ӷ�Ӧ����ʽ��______________________��
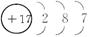
(2)D
(3)D
(4)���ⶨ��D
(1)![]() MnO2+4H++2Cl-
MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
(2)����
(3)H2O2+H2S![]() S��+2H2O
S��+2H2O
(4)H2O2![]() H++
H++![]() BaO2+H2SO4
BaO2+H2SO4![]() BaSO4��+H2O2
BaSO4��+H2O2
Na2O2+2H2O![]() 2NaOH+H2O2 ǰ��������η�Ӧ������������Σ�������ǿ�������ᣬ����������Na2O2��ˮ�ⷴӦ
2NaOH+H2O2 ǰ��������η�Ӧ������������Σ�������ǿ�������ᣬ����������Na2O2��ˮ�ⷴӦ
��������A�����������ǵ��Ӳ�����3��֪AΪO����CΪS����B���������Ϊx����x-(8-x)=6��x=+7��ͬCͬ����ΪCl��D��A��B��ͬ���ڣ�ֻ��ΪH.H2O2������������IJ��غϣ���Ϊ���Է��ӣ�H2O2Ϊ��Ԫ���ᣬӦ�ֲ�����.


