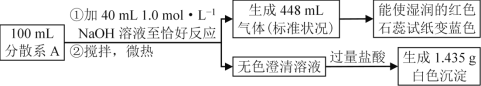
ЬтФПФкШн
ЁОЬтФПЁПЪЕбщЪвРћгУпЛрЋМзШЉЮЊдСЯжЦБИпЛрЋМзДМгыпЛрЋМзЫсЁЃ
ЂёЁЂжЦБИдРэЃК
2![]() ЃЋNaOH
ЃЋNaOH![]() ЃЋ
ЃЋ![]()
![]() ЃЋHCl
ЃЋHCl![]() ЃЋNaCl
ЃЋNaCl
ЂђЁЂЪЕбщВНжш
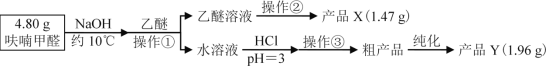
ЂѓЁЂЯрЙиаХЯЂ
пЛрЋМзШЉ | пЛрЋМзДМ | пЛрЋМзЫс | ввУб | |
ШлЕу/Ёц | Ѓ36.5 | Ѓ29 | 133 | Ѓ116.3 |
ЗаЕу/Ёц | 161.7 | 170 | 231 | 34.5 |
ЫЎШмад | ЮЂШм | ЮЂШм | ПЩШм | ВЛШм |
ЯрЖдЗжзгжЪСП | 96 | 98 | 112 | 74 |
ЂєЁЂЪЕбщзАжУ
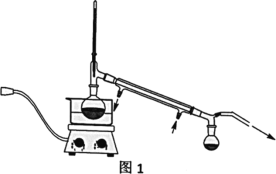
VЁЂЗжЮігыЫМПМЃЌЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉВйзїЂйУћГЦ___ЃЛВњЦЗYЮЊ___ЁЃ
ЃЈ2ЃЉВйзїЂкЕФзАжУШчЭМ1ЫљЪОЃЌЪеМЏВњЦЗXЪБЮТЖШМЦЕФЖСЪ§гІПижЦдк90ЁцзѓгвЃЌЦфдвђЪЧ___ЁЃ
ЃЈ3ЃЉВйзїЂйЫљЕУЫЎШмвКЃЌМгбЮЫсаыПижЦpHЮЊ2ЁЋ3ЃЌpHЃМ3ЕФРэгЩЪЧ___ЃЛПижЦШмвКpHЪБЃЌгІбЁдёЕФжИЪОМСЪЧ__ЁЃ
ЃЈ4ЃЉДжВњЦЗYДПЛЏЙ§ГЬгУЭМ2зАжУНјааШШЙ§ТЫЃЌОпЬхВйзїЃКЯђЭТЉЖЗжаМгШШЫЎЁњ___ЁњВ№зАжУЁЃЩцМАВйзїЫГађзюКЯРэЕФбЁЯюЁЃ
AЃЎМгШШТЉЖЗжЇЙмЁњЗХШыЖЬОБТЉЖЗЁњЗХШыТЫжНЁњЗХНгвКЩеБЁњЕЙШыШШЕФД§ТЫвК
BЃЎЗХШыЖЬОБТЉЖЗЁњЗХНгвКЩеБЁњМгШШТЉЖЗжЇЙмЁњЗХШыТЫжНЁњЕЙШыШШЕФД§ТЫвК
CЃЎЗХШыЖЬОБТЉЖЗЁњЗХШыТЫжНЁњМгШШТЉЖЗжЇЙмЁњЗХНгвКЩеБЁњЕЙШыШШЕФД§ТЫвК
DЃЎЗХШыЖЬОБТЉЖЗЁњЗХШыТЫжНЁњЗХНгвКЩеБЁњЕЙШыШШЕФД§ТЫвКЁњМгШШТЉЖЗжЇЙм
ЃЈ5ЃЉЙВЯћКФ30mLнЭШЁМСввУбЃЌДгнЭШЁаЇЙћНЧЖШЫМПМЃЌЯТСа4жжнЭШЁЗНЪНзюКЯРэЕФЪЧ__ЁЃ
AЃЎ30mLЁЂ0mLЁЂ0mL BЃЎ10mLЁЂ10mLЁЂ10mL
CЃЎ15mLЁЂ10mLЁЂ5 mL DЃЎ5mLЁЂ10mLЁЂ15mL
ЃЈ6ЃЉМЦЫуВњЦЗYЕФВњТЪІи(Y)ЃН___ЁЃ
ЁОД№АИЁПнЭШЁЗжвК пЛрЋМзЫс МѕбЙеєСѓБупЛрЋМзДМЗаЕуНЕЕЭЃЌЕЭгк170Ёц(дМ90Ёц)ЪБЗаЬкЪеМЏЃЌЗРжЙЮТЖШЙ§Ипв§Ц№пЛрЋМзДМНсЙЙЦЦЛЕ ПижЦpHЃМ3ЃЌЪЙпЛрЋМзЫсФЦЭъШЋзЊЛЏЮЊпЛрЋМзЫсЖјЮіГі МзЛљГШ(ЛђИеЙћКь) A B 70%
ЁОНтЮіЁП
пЛрЋМзШЉдкМюадЬѕМўЯТЗЂЩњЦчЛЏЗДгІЩњГЩ![]() КЭ
КЭ![]() ЃЌ
ЃЌ![]() взШмгкввУбЃЌМгШыввУбнЭШЁЗжвКЃЌЕУЕН
взШмгкввУбЃЌМгШыввУбнЭШЁЗжвКЃЌЕУЕН![]() ЕФввУбШмвККЭ
ЕФввУбШмвККЭ![]() ЕФЫЎШмвКЃЛ
ЕФЫЎШмвКЃЛ![]() ЕФввУбШмеєСѓЕУЕН
ЕФввУбШмеєСѓЕУЕН![]() ЃЛ
ЃЛ![]() ЕФЫЎШмвКМгШыбЮЫсЃЌЕїНкpH=3ЃЌЩњГЩ
ЕФЫЎШмвКМгШыбЮЫсЃЌЕїНкpH=3ЃЌЩњГЩ![]() ЮіГіЃЌдйгУжиНсОЇЗЈЬсДП
ЮіГіЃЌдйгУжиНсОЇЗЈЬсДП![]() ЁЃ
ЁЃ
ИљОнвдЩЯЗжЮіЃЌЃЈ1ЃЉВйзїЂйЪЧМгШыввУбЃЌЗжРы![]() ЕФввУбШмвККЭ
ЕФввУбШмвККЭ![]() ЕФЫЎШмвКЃЌУћГЦнЭШЁЗжвКЃЛ
ЕФЫЎШмвКЃЌУћГЦнЭШЁЗжвКЃЛ![]() ЕФЫЎШмвКМгШыбЮЫсЃЌЕїНкpH=3ЃЌЩњГЩ
ЕФЫЎШмвКМгШыбЮЫсЃЌЕїНкpH=3ЃЌЩњГЩ![]() ЮіГіЃЌЫљвдВњЦЗYЮЊпЛрЋМзЫсЁЃ
ЮіГіЃЌЫљвдВњЦЗYЮЊпЛрЋМзЫсЁЃ
ЃЈ2ЃЉВйзїЂкЮЊеєСѓзАжУЃЌЗРжЙЮТЖШЙ§Ипв§Ц№пЛрЋМзДМНсЙЙЦЦЛЕЃЌЭЈЙ§МѕбЙеєСѓБупЛрЋМзДМЗаЕуНЕЕЭЃЌЕЭгк170Ёц(дМ90Ёц)ЪБЗаЬкЪеМЏпЛрЋМзДМЃЌЫљвдЮТЖШМЦЕФЖСЪ§гІПижЦдк90ЁцзѓгвЁЃ
ЃЈ3ЃЉВйзїЂйЫљЕУЫЎШмвКЃЌМгШыбЮЫсЃЌПижЦpHЃМ3ЃЌЪЙпЛрЋМзЫсФЦЭъШЋзЊЛЏЮЊпЛрЋМзЫсЖјЮіГіЃЌЫљвдвЊПижЦpHЃМ3ЃЛМзЛљГШдкpHЃМ3.1ЪБЃЌГЪКьЩЋЃЌЫљвдПижЦШмвКpHЪБгІбЁдёМзЛљГШзїжИЪОМСЁЃ
ЃЈ4ЃЉЮЊЗРжЙпЛрЋМзЫсНсОЇЮіГіЃЌДжВњЦЗYДПЛЏЙ§ГЬгУШШЙ§ТЫЃЌОпЬхВйзїЃКЯђЭТЉЖЗжаМгШШЫЎЁњМгШШТЉЖЗжЇЙмЁњЗХШыЖЬОБТЉЖЗЁњЗХШыТЫжНЁњЗХНгвКЩеБЁњЕЙШыШШЕФД§ТЫвКЁњВ№зАжУЁЃЙЪбЁAЃЛ
ЃЈ5ЃЉДгнЭШЁаЇЙћНЧЖШЫМПМЃЌгУ30mLнЭШЁМСввУбЃЌзюКУЗжЖрДЮЕШСПнЭШЁЃЌКЯВЂнЭШЁвКЁЃЙЪбЁBЁЃ
ЃЈ6ЃЉпЛрЋМзШЉЕФЮяжЪЕФСПЪЧ![]() ЃЌИљОн2
ЃЌИљОн2![]() ЃЋNaOH
ЃЋNaOH![]() ЃЋ
ЃЋ![]() ЁЂ
ЁЂ![]() ЃЋHCl
ЃЋHCl![]() ЃЋNaClЃЌЩњГЩпЛрЋМзЫсЕФРэТлВњСПЪЧ0.025molЃЌжЪСПЪЧ0.025molЁС112g/mol=2.8gЃЌЪЕМЪЩњГЩпЛрЋМзЫсЕФжЪСПЪЧ1.96gЃЛВњЦЗYЕФВњТЪІи(Y)ЃН
ЃЋNaClЃЌЩњГЩпЛрЋМзЫсЕФРэТлВњСПЪЧ0.025molЃЌжЪСПЪЧ0.025molЁС112g/mol=2.8gЃЌЪЕМЪЩњГЩпЛрЋМзЫсЕФжЪСПЪЧ1.96gЃЛВњЦЗYЕФВњТЪІи(Y)ЃН![]() 70%ЁЃ
70%ЁЃ

ЁОЬтФПЁПвбжЊЗДгІЃК4HCl(g)+O2(g)ЈT2Cl2(g)+2H2O(g)ЃЌвЛЖЈЬѕМўЯТВтЕУЗДгІЙ§ГЬжаn(Cl2)гыЪБМфЕФЙиЯЕШчБэЫљЪОЃКдђ4ЁЋ6minФкгУO2ЕФЮяжЪЕФСПБфЛЏБэЪОЕФЗДгІЫйТЪЪЧ(ЁЁЁЁ)
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
n(Cl2)/mol | 0 | 1.2 | 2.6 | 4.4 | 5.4 | 6.0 |
A.7.2molmin-1B.8.0molmin-1C.0.9molmin-1D.0.45molmin-1