��Ŀ����
��10�֣�������Mg������100mL 1.5mol��L��1ϡH2SO4�У���Ӧ��������ȥ����Mg�ۣ���Һ��t��������������Һ����Ϊ72.0gʱ��ʼ����MgSO4��xH2O���壬����������12.3gʱ��ʣ����Һ48 .0g��ͨ��������ش��������⣨��д��������̣���
.0g��ͨ��������ش��������⣨��д��������̣���
��1�����ɱ�״���� �����������
�����������
�� 2����ʼ����MgSO4��xH2O����ʱ��Һ������������
2����ʼ����MgSO4��xH2O����ʱ��Һ������������
��3��MgSO4��xH2O�е�xֵ��
 .0g��ͨ��������ش��������⣨��д��������̣���
.0g��ͨ��������ش��������⣨��д��������̣�����1�����ɱ�״����
 �����������
�������������
 2����ʼ����MgSO4��xH2O����ʱ��Һ������������
2����ʼ����MgSO4��xH2O����ʱ��Һ��������������3��MgSO4��xH2O�е�xֵ��
��1��3.36L ��2��25.0%��3��7
n��H2SO4��=0.1L��1.5mol��L��1=0.15mol
��1��Mg �� H2SO4 = MgSO4 �� H2��
0.15mol 0.15mol 0.15mol
V��H2��=22.4L��mol��1��0.15mol=3.36L
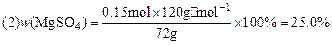
��3��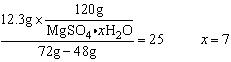
��1��Mg �� H2SO4 = MgSO4 �� H2��
0.15mol 0.15mol 0.15mol
V��H2��=22.4L��mol��1��0.15mol=3.36L
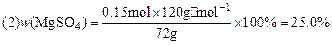
��3��
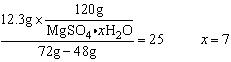

��ϰ��ϵ�д�
�����Ŀ
 a2O2��CaCl2��KOH��NaF�У�ֻ���й��ۼ���������_________________��ֻ�������Ӽ���������___________���Ⱥ������Ӽ����ֺ��й��ۼ���������____________��
a2O2��CaCl2��KOH��NaF�У�ֻ���й��ۼ���������_________________��ֻ�������Ӽ���������___________���Ⱥ������Ӽ����ֺ��й��ۼ���������____________��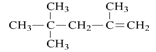 ������Ϊ��2,2,4-����-4-��ϩ
������Ϊ��2,2,4-����-4-��ϩ ����B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
����B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��