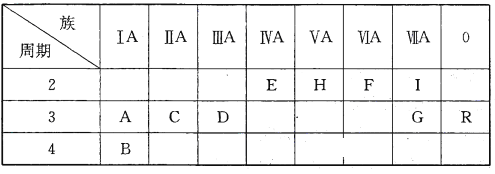
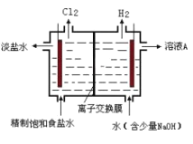
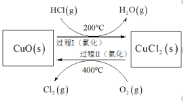
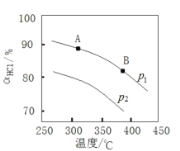
��Ŀ����
����Ŀ��ʵ��������Na2SO4��10H2O����������100mL0.2mol/L��Na2SO4��Һ���Իش����и��⣺
��1����Ҫ��������ƽ����__Na2SO4��10H2O���壻
��2�����������У�����Ҫ�õ�����(�����)___��
��©�� ��180mL����ƿ ���ձ� �ܲ����� ��ҩ�� ��������ƽ ��������
��Ҫʵʩ���ƣ������������⣬��ȱ�IJ���������__��
��3������ƿ�ϳ��п̶����Ӧ����_����ʹ��ǰ������__��
��4�����ƹ��������²�����
�ٳ��� ����Һ ����ȴ ��ϴ�� �ݶ��� ���ܽ� ��ҡ��
����ȷ�IJ���˳��Ӧ��___����������������ڣ���
������ ������ ������ ������ ������ ������
��5��������������лᵼ�¸�ʵ�������Ƶ���ҺŨ��ƫ�͵���___������ţ���
�ٳ���ʱ����������ŷ���
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
��Na2SO410H2O��������Һǰʧȥ�˲��ֵĽᾧˮ
�ܶ���ʱ�۲�Һ��������ͼ��ʾ�����ӣ�
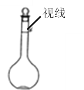
�ݶ��ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ���μӼ���ˮ���̶ȴ�
���𰸡�6.4g �٢ڢ� ��ͷ�ιܡ�100mL����ƿ �ݻ����¶� �������ƿ�Ƿ�����Լ�ƿ�����Ƿ�©ˮ �ޢۢڢܢ� �٢�
��������
(1)ʵ�������Na2SO4��10H2O��������ʵ���Ϊ0.1L��0.2mol/L=0.02mol������Ϊ��322g/mol��0.02mol=6.4g��������Ҫ��������ƽ����6.4g Na2SO4��10H2O���壬�ʱ����Ϊ��6.4g��
(2)����һ�����ʵ���Ũ�ȵ�ʵ�飬��Ҫ�õ�100mL����ƿ���ձ�����������ҩ�ס�������ƽ����ͷ�ιܵ�����������Ҫ���ǣ�©����180mL����ƿ�������ܣ��ʱ����Ϊ���٢ڢߣ���ͷ�ιܡ�100mL����ƿ��
(3)����ƿ�Ǿ������������п̶����Ӧ�����ݻ����¶ȣ���ʹ��ǰ�����ȼ������ƿ�Ƿ�����Լ�ƿ�����Ƿ�©ˮ���ʱ����Ϊ���ݻ����¶ȣ��������ƿ�Ƿ�����Լ�ƿ�����Ƿ�©ˮ��
(4)���ƹ��̵���ȷ����˳��Ϊ���������ܽ�����ȴ����Һ��ϴ����������ҡ�ȣ��ʱ�����ȷ��Ϊ���ޢۢڢܢݣ�
(5)�ٳ���ʱ����������ŷ��ˣ��ᵼ�³�������Ʒ�������٣�����Ũ��ƫ�ͣ��ٷ������⣻
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ���ʹ��Һ���ƫС������Ũ��ƫ�ڲ��������⣻
��Na2SO410H2O��������Һǰʧȥ�˲��ֵĽᾧˮ����ʹ������Na2SO4�������࣬����Ũ��ƫ�۲��������⣻
�ܶ���ʱ���ӣ���ʹ��Һ�����ƫС������Ũ��ƫ�ܲ��������⣻
�ݶ��ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ���μӼ���ˮ���̶ȴ�����ʹ��Һ���ƫ����Ũ��ƫС���ݷ������⣻
�ʱ����Ϊ���٢ݡ�

����Ŀ�������й�ʵ�������ѡ��Ͳ���ʵ���������ȷ���ǣ� ��
ѡ�� | ʵ�� | ��ѡ��Ҫ��������̨�Ⱥ��� | ���ֲ��� |
A | ��Ũ��������480mL0.1mol/L������Һ | 500mL����ƿ���ձ�������������Ͳ����ͷ�ι� | ����ϡ�����õ�������Һ����ת����500mL����ƿ������ˮ���̶����� |
B | ��ʳ��ˮ�еõ�NaCl���� | �������ƾ��ơ��������������ǡ����ż� | �������������������ʱ��ֹͣ���ȣ��������ȼ������� |
C | �ӵ�����Ȼ�̼��Һ�еõ��� | ������ƿ���ƾ��ơ��¶ȼơ������ܡ���ƿ��ţ�ǹ� | �¶ȼ�ˮ�������ҺҺ������ |
D | ��������ȡ��ˮ�е�Br2 | ��Һ©�����ձ� | ����ʱ�²�Һ��ӷ�Һ©���¿ڷų����ϲ�Һ����Ͽڵ��� |
A.AB.BC.CD.D
����Ŀ������ʵ��������ʵ���������ƥ����ǣ� ��
ʵ����� | ʵ������ | |
A | ��þ����ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в���Ũ�̲��к�ɫ�������� |
B | ��ʢ�и������������Һ���Թ���ͨ���� ������ϩ���� | ��Һ����ɫ����ȥ�����ú���Һ�ֲ� |
C | ��ʢ�б��������������Һ���Թ��еμ�ϡ���� | �д̼�����ζ�����������Һ����� |
D | ��ʢ��FeCl3��Һ���Թ��мӹ������ۣ�������1��KSCN��Һ | ��ɫ����ʧ����KSCN����Һ��ɫ���� |
A. AB. BC. CD. D
����Ŀ��ijʵ��С��̽��![]() ��
��![]() �ķ�Ӧ���ɡ�ʵ�����������������
�ķ�Ӧ���ɡ�ʵ�����������������
ʵ�鼰�Լ� | ��� | ��ɫ | �Թ�����Һ��ɫ | ����KI�Լ���ɫ |
| 1 | 0.5mL | dz��ɫ | ��ɫ |
2 | 0.20mL | ���ɫ | ��ɫ | |
3 | 0.25mL | dz��ɫ | ��ɫ | |
4 | 0.30mL | ��ɫ | ��ɫ |
��1��ȡʵ��2�����Һ����������ʵ�飺

������ͬѧ���飬������ɫ������![]() ��д������0.20mL
��д������0.20mL ![]() ����Һ��
����Һ��![]() ��
��![]() ������Ӧ�����ӷ���ʽ��____________��
������Ӧ�����ӷ���ʽ��____________��
��2���������ϣ�һ�������£�![]() ��
��![]() �����Ա�������
�����Ա�������![]() ��
��
�������裺![]() ��Һ�������ӵ�����Һ��ɫ��ԭ���ǹ�����
��Һ�������ӵ�����Һ��ɫ��ԭ���ǹ�����![]() ��Һ�루1���еķ�Ӧ���������Ӧ��ͬʱ����
��Һ�루1���еķ�Ӧ���������Ӧ��ͬʱ����![]() ������ʵ�飺
������ʵ�飺
��ȡ����ʵ��4�е���ɫ��Һ��������ʵ�飬��һ����֤���к���![]() ��
��

�����Լ�X������_________������ĸ��ţ���
a ��ˮ b ![]() ��Һ c
��Һ c ![]() ��Һ d
��Һ d ![]() ��Һ
��Һ
������ͬѧ�������ͨ����ʪ����![]() ��ֽ������������˵������
��ֽ������������˵������![]() ��������_______���㲹��������ʵ�飺��ʵ��4�е�ʪ�����
��������_______���㲹��������ʵ�飺��ʵ��4�е�ʪ�����![]() ��ֽ�滻Ϊ________���ڵμ�
��ֽ�滻Ϊ________���ڵμ�![]() ��Һ����________����һ����֤ʵ��4��������
��Һ����________����һ����֤ʵ��4��������![]() ��
��