��Ŀ����
��6�֣�A��B��C����Ԫ�ص�ԭ�Ӿ�����ͬ�ĵ��Ӳ�������B�ĺ˵������A��1��Cԭ�ӵĵ���������Bԭ�ӵĵ���������4�� 1molA�ĵ��ʸ��������ᷴӦ�����û�����״����22.4L��H2����ʱAת��Ϊ����ԭ�Ӿ�����ͬ���Ӳ�ṹ�����ӡ���ش�
��1���õ���ʽ��ʾA��C��ɵĻ�������γɹ���Ϊ______________________��
��2��B���ӵĵ���ʽ�� ����B�����Ӿ�����ͬ�������ķ����У���һ�ַ���
�������ữ�������Σ��÷��ӵĵ���ʽ�� ��
���ڱ�����C�������ڵ�ͬ��Ԫ���γɵ���̬�⻯���У��е���ߵ��� ������
�⻯�ﻯѧʽ����ԭ���� ��
д��B����������ˮ�����C����������ˮ����֮�䷴Ӧ�����ӷ���ʽ����
���漰�ĺ�CԪ�ص����ʾ�������ˮ���� ��
��1�� MgCl2 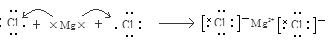 ;��2�� Al3+ ;
;��2�� Al3+ ; 
��3�� HF HF����֮�������� ��4�� Al(OH)3+3H+=Al3++3H2O
��������������ṹ�����֪��A��Mg��B��Al��C��Cl����1���õ���ʽ��ʾA��C��ɵĻ�����MgCl2���γɹ���Ϊ�� ;��2��B���ӵĵ���ʽ��Al3+;��B�����Ӿ�����ͬ�������ķ����У���һ�ַ��ӿ������ữ�������Σ��÷�����NH3,���ĵ���ʽ��
;��2��B���ӵĵ���ʽ��Al3+;��B�����Ӿ�����ͬ�������ķ����У���һ�ַ��ӿ������ữ�������Σ��÷�����NH3,���ĵ���ʽ�� ����3�����ڱ�����C�������ڵ�ͬ��Ԫ���γɵ���̬�⻯���У��е���ߵ���HF��ԭ������HF����֮���������������˷���֮��������������4��B����������ˮ����Al(OH)3��C����������ˮ����HClO4֮�䷴Ӧ�����ӷ���ʽ��Al(OH)3+3H+=Al3++3H2O��
����3�����ڱ�����C�������ڵ�ͬ��Ԫ���γɵ���̬�⻯���У��е���ߵ���HF��ԭ������HF����֮���������������˷���֮��������������4��B����������ˮ����Al(OH)3��C����������ˮ����HClO4֮�䷴Ӧ�����ӷ���ʽ��Al(OH)3+3H+=Al3++3H2O��
���㣺����Ԫ�ص��ƶϡ�Ԫ���γɵĻ���������ʡ����ʼ��γɹ��̵ĵ���ʽ��ʾ�����ӷ���ʽ����д��

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�A��B�Ƕ����ڵ�����Ԫ�أ����ǵ�����A����B2��������ͬ�ĺ�����Ӳ�ṹ������˵������ȷ���ǡ�
| A��ԭ������A��B | B����������A��B |
| C��ԭ�Ӱ뾶A��B | D�����Ӱ뾶A����B2�� |
Ԫ�����ڱ���Ԫ����������ѧϰ���о�������ʵ�����к���Ҫ�����á��±��г��ˢ١������Ԫ�������ڱ��е�λ�á�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | | �� | | |
| 3 | �� | �� | �� | | | | �� | �� |
| 4 | �� | | | | | | �� | |
��2���ڢ١��ڡ�������Ԫ�ص��������Ӧ��ˮ�����У�������ǿ���� (�ѧʽ)��
��3���١��ڡ�������Ԫ�ذ����Ӱ뾶�ɴ�С��˳������Ϊ (�����ӷ���)��
��4����Ԫ���γɵľ���ǿ�����Ե��⻯�����ʽ�� �� ��Ԫ����һ���⻯���ڳ������� �ڷ�����Ӧ�Ļ�ѧ����ʽΪ ��
(16��)�±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһ��ѧԪ�ء�
| | | | |||||||||||||||
| | | | | h | | | | | |||||||||
| a | c | f | | i | | | m | ||||||||||
| | | | e | | | | | | | | | | g | | k | | |
| | d | | | | | | | | | | | | | | | | |
��1����������Ԫ���У����ڶ�����Ԫ�ص��� ��дԪ�ط��ţ�,
eԪ����Ԫ�����ڱ��е�λ���� ���ڣ� �塣
��2������ ��Ԫ�صĵ��ʿ��ܶ��ǵ�������塣
A��a, c, h B �� i ,g, k C��c, h, m D�� d, e, f
��3��iԪ������ ���������ǽ�����Ԫ�أ�������������ϼ���
�����ϼ��� �����ܸ�������Ӧ������һ����ˮ�Ժ�ǿ�����ʣ�����������ʵ�����ﳣ�����������д������������Ӧ�Ļ�ѧ����ʽ�� ��