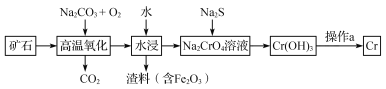
��Ŀ����
����Ŀ�����������ǿ�ѧѧϰ����Ҫ����֮һ������Ԫ�صIJ������ʹ����������£�
|
|
|
|
|
�����۵�/�� | -218,4 | 113 | �� | 450 |
���ʷе�/�� | -183 | 444.6 | 685 | 989 |
��Ҫ���ϼ� | -2 | -2��+4��+6 | -2��+4��+6 | �� |
ԭ�Ӱ뾶/nm | 0.074 | 0.102 | �� | 0.136 |
�������⻯�ϵ����׳̶� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�� |
������ϱ������Լ����ݱ仯���ƣ��ش��������⣺
��1�������۵㷶Χ�����ǣ�________________��
��2���ڣ�Te�����ܵĻ��ϼ��У�___________________��
��3������Se����ԭ�Ӱ뾶������_______________________֮�䡣
��4���ӵ������⻯�ϵ����׳̶ȿ����Ʋ����O��S��Se��Te�ķǽ�������_________(��ǿ������)��
���𰸡���1��113��--450��֮�䣨2��-2,+4��+6��3�� 0.102��0.136֮�䣨4������
���������������������1���ɱ���֪���������ڣ����ʵ��۵������ߣ����������۵㷶Χ������113����450����
��2�������Ļ��ϼ���-2��+4��+6���ڵ������������ƣ������ڵĻ��ϼۿ�����-2��+4��+6��
��3������Se����ԭ�Ӱ뾶����S��Te֮�䣬������Se����ԭ�Ӱ뾶Ϊ0.102��0.136��
��4��������������ӦԽ���ף�Ԫ�صķǽ�����Խǿ���ɷ�Ӧ������֪��O��S��Se��Te�ķǽ�����������

 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�����Ŀ�������������ǻ�ѧ�빤ҵ�����ù㷺�����ʡ�

��1����С�����õ��ԭ�����������ͼװ�ý���H2��ԭNO��ʵ��(�����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ������ٱ�Ĥ���缫)�����缫B��____�����ٵ缫A�ĵ缫��ӦʽΪ_____________��
��2������ҵ��ˮ�е�NO2- ��Ũ��ԼΪ1.0��10-4 mol��L-1 ��ȡ��ҵ��ˮ5 mL ���Թ��У��μ�2��0.1 mol��L-1 ����������Һ���ܷ�����������ͨ������˵����(ע��1mL��Һ��20�μƣ�Ksp(AgNO2)=2��10-8)
��3����֪�������ݣ�H-H 436��S=S 255��H-S 339����λkJ/mol��������Ȼ�ѧ����ʽ2H2(g) + S2(g) = 2H2S(g)�Ħ�H=___________
��4����һ���������İ���������������Ƶ��ܱ����������(��������������䣬��������������Բ���)���ں㶨�¶���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4(s)![]() 2NH3(g)��CO2(g)��
2NH3(g)��CO2(g)��
ʵ���ò�ͬ�¶��µ�ƽ�����������±���
�¶�(��) | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 |
ƽ��������Ũ��(��10��3mol/L) | 2.4 | 3.4 | 4.8 | 6.8 | 9.4 |
���ݱ������ݣ���ʽ����25.0��ʱ�ķֽ�ƽ�ⳣ����__________________________��
��֪��NH2COONH4��2H2O![]() NH4HCO3��NH3��H2O�����о�С��ֱ������ݲ�ͬ��ʼŨ�ȵİ����������Һ�ⶨˮ�ⷴӦ���ʣ��õ�c(NH2COO��)��ʱ��仯������ͼ��ʾ��
NH4HCO3��NH3��H2O�����о�С��ֱ������ݲ�ͬ��ʼŨ�ȵİ����������Һ�ⶨˮ�ⷴӦ���ʣ��õ�c(NH2COO��)��ʱ��仯������ͼ��ʾ��

����ͼ����Ϣ�����˵��ˮ�ⷴӦ�������¶����߶�����_____________________��
����Ŀ����X��Y��Z��T��U���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ��������ʾ����Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ����������T2X(��ɫ����)��T2X2(����ɫ����)���ֻ����U������Z������ȼ��ʱ������ɫ���棬�������ˮ��Һ��ʹʯ����Һ��졣
X | |
Y | Z |
��1����Ԫ�صķ����ǣ�Z________��T________
��2��Yԭ�ӵĽṹʾ��ͼΪ____________________,U2X�ĵ���ʽ
��3��YX2��U2Y��Ӧ�Ļ�ѧ����ʽΪ____________________________��������������____________����������Ԫ����____________��