��Ŀ����
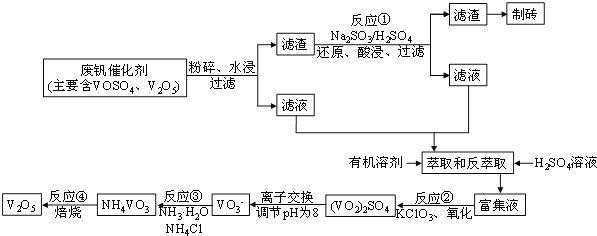
����Ŀ������ZnO�׳�п�ף��ܸĽ������Ļ�ѧ�ȶ��ԣ��������������ֲ�������ҵ���ɴ�п![]() ��FeO��CuO��
��FeO��CuO��![]() �Ʊ�ZnO����ȡ����롢�������ӡ��кͳ���������Ȳ��裬�������£�
�Ʊ�ZnO����ȡ����롢�������ӡ��кͳ���������Ȳ��裬�������£�

![]() ������������X�����������е� ________________
������������X�����������е� ________________
A.ZnO B.NaOH C.Zn(OH)2 D.Na2CO3
![]() ��֪ZnOΪ���������������������Һ��Ӧ�����ɿ����Ե�п����
��֪ZnOΪ���������������������Һ��Ӧ�����ɿ����Ե�п����![]() ��д����Ӧ�����ӷ���ʽ_________________________��
��д����Ӧ�����ӷ���ʽ_________________________��
![]() �����
д������![]() ʱ������Ӧ�����ӷ���ʽ _________________________________��
ʱ������Ӧ�����ӷ���ʽ _________________________________��
![]() ��п�۹��˵õ���������Ҫ�ɷ���_________
��п�۹��˵õ���������Ҫ�ɷ���_________
![]() ���������ж���õ��˹��˲�������д������ʱ�õ��IJ���������_________��
���������ж���õ��˹��˲�������д������ʱ�õ��IJ���������_________��
![]() ������ҺA�����ʵ������ӵķ�����________________________________��
������ҺA�����ʵ������ӵķ�����________________________________��
![]() ȡ��ʽ̼��пˮ����
ȡ��ʽ̼��пˮ����![]() ���������������ɶ�����̼
���������������ɶ�����̼![]() �����
�����![]() ���ܽ���������Ȼ���
���ܽ���������Ȼ���![]() �����ü�ʽ������Ԫ�ص���������Ϊ
�����ü�ʽ������Ԫ�ص���������Ϊ![]() �����Ʋ�ü���̼��пˮ����Ļ�ѧʽ��__________________��
�����Ʋ�ü���̼��пˮ����Ļ�ѧʽ��__________________��
���𰸡�![]()
![]()
![]()
![]() п��ͭ �ձ���©���������� ȡ
п��ͭ �ձ���©���������� ȡ![]() ���Թ��У��������ữ���ٵμ��Ȼ�����Һ�������ɰ�ɫ������˵���������������
���Թ��У��������ữ���ٵμ��Ȼ�����Һ�������ɰ�ɫ������˵��������������� ![]()
��������
��ZnO�������ܽ⣬Ȼ����ˣ�����Һ�м���������⣬���������������������ӣ��ٵ���pHֵ��ʹ�����ӳ���������pHֵʱ��������ʼ������ᷴӦ��Ҫ�����������ʣ�����X����Ϊ����п��������п�ȣ�������Һ��PH��ʹ�����ӿ�ʼ����������Һ�м���п�ۣ�����Һ��ͭ���Ӻ����������ã����ˣ�����Һ�м���̼�����ʹ������������������õ���ʽ̼��п��
![]() ��������ͼ��֪��������X��Ŀ����ʹ��Ԫ���γɳ���������ZnO��
��������ͼ��֪��������X��Ŀ����ʹ��Ԫ���γɳ���������ZnO��![]() �ܴٽ������ӵ�ˮ��ʹ֮�γɳ�������AC��ȷ������NaOH��
�ܴٽ������ӵ�ˮ��ʹ֮�γɳ�������AC��ȷ������NaOH��![]() �����ӳ�����ͬʱ��п��ͭҲ��ת��Ϊ��������BD���ʴ�Ϊ��AC��
�����ӳ�����ͬʱ��п��ͭҲ��ת��Ϊ��������BD���ʴ�Ϊ��AC��
![]() ��֪ZnOΪ���������������������Һ��Ӧ���ɿ����Ե�п����
��֪ZnOΪ���������������������Һ��Ӧ���ɿ����Ե�п����![]() �����ӷ���ʽΪ
�����ӷ���ʽΪ![]()
![]() ��
��
![]() ��
��![]() �ǽ���������Ϊ��������������Ӧ�����ӷ���ʽ
�ǽ���������Ϊ��������������Ӧ�����ӷ���ʽ![]() ��
��
![]() ��ȥ
��ȥ![]() ������Һ�м���Zn��Ŀ���dz�ȥ��Һ�к��е�ͭ���ӣ�п�û���ͭ����пӦ�������������õ������У�п��ͭ��
������Һ�м���Zn��Ŀ���dz�ȥ��Һ�к��е�ͭ���ӣ�п�û���ͭ����пӦ�������������õ������У�п��ͭ��
![]() ����ʱ�õ��IJ��������У��ձ���©������������
����ʱ�õ��IJ��������У��ձ���©������������
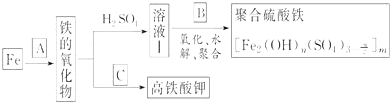
![]() �������̷�����̼�����ϴ�ӳ����������ᷴӦ��������泥�����A����Ҫ���е�������
�������̷�����̼�����ϴ�ӳ����������ᷴӦ��������泥�����A����Ҫ���е�������![]() �����Լ�����������ӣ���������ӵļ��鷽��Ϊ��ȡ
�����Լ�����������ӣ���������ӵļ��鷽��Ϊ��ȡ![]() ���Թ��У��������ữ���ٵμ��Ȼ�����Һ��������ְ�ɫ������˵��������������ӣ�
���Թ��У��������ữ���ٵμ��Ȼ�����Һ��������ְ�ɫ������˵��������������ӣ�
![]() ��
�� ��
��![]() ��
��![]() ����
����![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��4��1��1���ʻ�ѧʽΪ��
��4��1��1���ʻ�ѧʽΪ��![]() ����
����![]() ��
��

����Ŀ����ҵ�Ͽ�������������װһ��װ�����ⶨ��������Ҫ�ɷ�FeS2���������������(����SO2��H2SO3�������ķ�Ӧ)��ʵ�����ȷ�����������£�
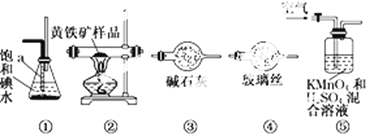
A�����Ӻ�װ�ã������װ�õ�������
B����ȡ��ϸ�Ļ�������Ʒ
C����2.0 g��ƷС�ĵط���Ӳ�ʲ�������
D����1 L/min�����ʹ������
E����Ӳ�ʲ������еĻ�������Ʒ���ȵ�800�桫850��
F����300 mL�ı��͵�ˮ����SO2�������ķ�Ӧ�ǣ�I2��SO2��2H2O = 2HI��H2SO4
G������Һ��CCl4��ȡ������
H��ȡ20.00mLG��������Һ����0.2000mol��L��1��NaOH����Һ�ζ����Իش�
��1������G��������Ҫ������______��Ӧȡ_______ (����������������)����Һ���к���ʵ�顣
��2��װ����ȷ������˳���� ![]()
![]()
![]() ��
�� ![]() (����)��______
(����)��______
��3��װ�â��и�����ص�������__________�������������������__________��
��4������H�еζ�ʱӦѡ��_____��ָʾ�������Ը���________�������жϵζ��Ѿ��ﵽ�յ㡣
��5���ٶ��������е����ڲ���E����ȫ��ת��ΪSO2�����ұ����͵�ˮ��ȫ���գ��ζ��õ����������±���ʾ��
�ζ����� | ����Һ�����/mL | NaOH����Һ�����/mL | |
�ζ�ǰ | �ζ��� | ||
��һ�� | 20.00 | 0.00 | 20.48 |
�ڶ��� | 20.00 | 0.22 | 20.20 |
������ | 20.00 | 0.36 | 20.38 |
���������Ʒ����Ԫ�ص���������Ϊ___________��
��6��Ҳ������������������������ⶨ�������к��������������������ַ����ⶨ���������װ�â���������Һ�м������������Լ�__________ ��
A.��������Һ B.�Ȼ�����Һ C.����ʯ��ˮ D.���Ը��������Һ