��Ŀ����
�����й�ʵ��������������ȷ���ǣ�������
| A������к͵ζ�ǰ��������ˮϴ�ӵζ��ܺ���ƿ�����ô�װ��Һ��ϴ |
| B�������£���0.1mol?L-1ijһԪ�ROH����Һ��pH=l��HCl��Һ�������ϣ����Ϻ���Һ��pH��7 |
| C�������£��ⶨij������Һ��pHʱ��ʹ��ʪ���pH��ֽ���pHһ���������� |
| D����������ƽ��ȡ1.06g��ˮ̼���ƣ�С��ת����250 mL����ƿ�У���������ˮ���̶��ߣ����Ƶõ�0.04mol?L-1 Na2CO3��Һ |
���㣺��ѧʵ�鷽��������
ר�⣺ʵ��������
������A����ƿ����Ҫ��ϴ��
B��pH=l��HCl��Һ��Ũ��Ϊ0.1mol/L����0.1mol/LROHΪǿ��������Ϻ�pH=7����Ϊ���������������ǿ�������Σ�pH��7��
C��ʹ��ʪ���pH��ֽ��һ������
D��������ƽ��ȡ1.06g��ˮ̼���ƣ������ܣ�
B��pH=l��HCl��Һ��Ũ��Ϊ0.1mol/L����0.1mol/LROHΪǿ��������Ϻ�pH=7����Ϊ���������������ǿ�������Σ�pH��7��
C��ʹ��ʪ���pH��ֽ��һ������
D��������ƽ��ȡ1.06g��ˮ̼���ƣ������ܣ�
���
�⣺A����ƿ����Ҫ��ϴ����ϴʱ�ζ����ƫ��������ˮϴ�ӵζ��ܺ����ô�װ��Һ��ϴ����A����
B��pH=l��HCl��Һ��Ũ��Ϊ0.1mol/L����0.1mol/LROHΪǿ��������Ϻ�pH=7����Ϊ���������������ǿ�������Σ�pH��7�������£���0.1mol?L-1ijһԪ�ROH����Һ��pH=l��HCl��Һ�������ϣ����Ϻ���Һ��pH��7����B��ȷ��
C��ʹ��ʪ���pH��ֽ��һ��������ⶨNaCl��������Һ��pH��Ӱ�죬���ⶨ���ԡ�������Һ��Ӱ�죬��C����
D��������ƽ��ȡ1.06g��ˮ̼���ƣ������ܣ�������ƽ��������ֻ��ȷ��0.1g����D����
��ѡB��
B��pH=l��HCl��Һ��Ũ��Ϊ0.1mol/L����0.1mol/LROHΪǿ��������Ϻ�pH=7����Ϊ���������������ǿ�������Σ�pH��7�������£���0.1mol?L-1ijһԪ�ROH����Һ��pH=l��HCl��Һ�������ϣ����Ϻ���Һ��pH��7����B��ȷ��
C��ʹ��ʪ���pH��ֽ��һ��������ⶨNaCl��������Һ��pH��Ӱ�죬���ⶨ���ԡ�������Һ��Ӱ�죬��C����
D��������ƽ��ȡ1.06g��ˮ̼���ƣ������ܣ�������ƽ��������ֻ��ȷ��0.1g����D����
��ѡB��
���������⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬���ջ�ѧ��Ӧԭ����ʵ���������Ϊ���Ĺؼ����漰�к͵ζ�ʵ�顢pH�ⶨ����Һ���Ƽ�����ϵļ���ȣ�ע��ʵ��������ԺͲ����Է�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ
�����£�������Һ�϶������Ե��ǣ�������
| A�����������Ӧ�ų����� |
| B����Һ�к���H+ |
| C�����������Ӧ�ų����� |
| D�������̪��Һ����Һ����ɫ |
һ���¶��£�ijһ�����ܱ��������п��淴Ӧ��A��g��+3B��g��?2C��g�����÷�Ӧ���е�һ���Ⱥ�ﵽ��ѧƽ��ı�־�ǣ�������
| A����λʱ��������a mol����A��ͬʱ����3a mol����B |
| B������C���������ʺͷֽ�������� |
| C��������������ܶȲ�����ʱ����ı� |
| D������A��B��C�ķ�����֮��Ϊ1��3��2 |
����ʵ���������ȷ���ǣ�������
| A�����������Ȼ���ҩƷʱ����ҩƷֱ�ӷ�����ƽ������ |
| B��ʵ���������������������ʱ�ȴ�ˮ�����Ƴ������ܣ���Ϩ��ƾ��� |
| C���������������ʱ����Ӧװ���е��Թܿ�Ӧ��������б |
| D����������ԭ����ͭʱ���������鴿����ֹͣ���Ⱥ�Ҫ����ͨ������ֱ����ȴ |
��ѧ����ᡢ����������������أ�����˵����ȷ���ǣ�������
| A��������ϩ������Ʒ������ʳƷ��װ |
| B�����ع��͡���ֹʳ�ã������������Ʒ��� |
| C��ú����������Һ���������仯��ת��Ϊ���ȼ�� |
| D����ά�ء���֬�������ʾ�����Ȼ�߷��ӻ����� |
���й����л��������������ȷ���ǣ�������
| A���������У����е���ԭ�Ӹ���һ����ż�� |
| B���û�ѧʽC4H10��ʾ������һ���Ǵ����� |
C��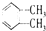 �� �� ��ͬ�����ʣ�˵����������̼̼������ͬ ��ͬ�����ʣ�˵����������̼̼������ͬ |
| D��ij�л�����ȫȼ��ֻ����CO2��H2O������л��ﲻһ������ |
�ڱȽϻ�ѧ��Ӧ����ʱ�����������õ��������Ϊ��������
| A��������������ϵ��ѹǿ |
| B����ɫ����dz |
| C�������������Ķ��� |
| D����Ӧ�ľ��ҳ̶� |
��ұ��ҵ�ϣ����õ�ⷨ�õ�K��Ca��Na��Mg��Al�Ƚ�������ԭ���ǣ�������
| A����Щ�����ܻ��� |
| B����������� |
| C����Щ�����������۵�� |
| D���ɱ��� |