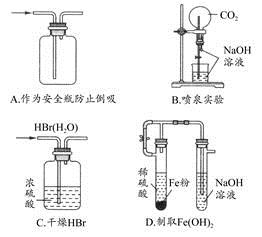
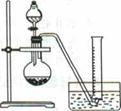
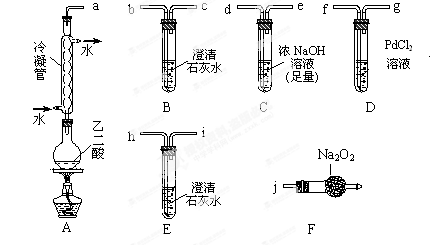
��Ŀ����
��ͭ��ʯ��Ҫ��������ͭ��Cu2S����������ʯ��SiO2����һ���Ի�ͭ��ʯΪԭ���Ʊ�����ͭ�Ĺ����������£�
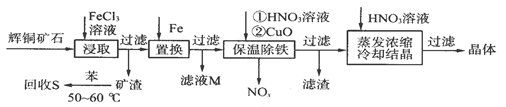
��д����ȡ������Cu2S�ܽ�����ӷ���ʽ��____________________��
�ƻ���S�������¶ȿ�����50��60��֮�䣬���˹�����͵�ԭ����_____________��
������NOx��������Ϻ�ͨ��ˮ�������������п�ѭ�����õ�һ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ___________________________������ҺM�м��루��ͨ�룩����__________������ĸ�����ʣ��õ���һ�ֿ�ѭ�����õ����ʡ�
a���� b������ c���������
�ȱ��³��������У�����CuO��Ŀ����________________________������Ũ��ʱ��Ҫ��HNO3��Һ������Һ��pH����������___________________________��

��д����ȡ������Cu2S�ܽ�����ӷ���ʽ��____________________��
�ƻ���S�������¶ȿ�����50��60��֮�䣬���˹�����͵�ԭ����_____________��
������NOx��������Ϻ�ͨ��ˮ�������������п�ѭ�����õ�һ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ___________________________������ҺM�м��루��ͨ�룩����__________������ĸ�����ʣ��õ���һ�ֿ�ѭ�����õ����ʡ�
a���� b������ c���������
�ȱ��³��������У�����CuO��Ŀ����________________________������Ũ��ʱ��Ҫ��HNO3��Һ������Һ��pH����������___________________________��
��1��Cu2S+4Fe3+=2Cu2++4Fe2++S
��2���¶ȹ��߱����ӷ����¶ȹ����ܽ�����С��
��3��4NOx+��5��2x��O2+2H2O= 4HNO3 b����4��������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3��������Cu2+��ˮ�⣨�������������ʣ�
��2���¶ȹ��߱����ӷ����¶ȹ����ܽ�����С��
��3��4NOx+��5��2x��O2+2H2O= 4HNO3 b����4��������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3��������Cu2+��ˮ�⣨�������������ʣ�
��1��Fe3�� ����������Cu2S���������������ʽΪ��Cu2S+4Fe3+=2Cu2++4Fe2++S���𰸣�Cu2S+4Fe3+=2Cu2++4Fe2++S����2�����е�Ƚϵͣ��¶ȹ��߱����ӷ����ӷ�Ӧ����ѧ�������¶ȹ����ܽ�����С���𰸣��¶ȹ��߱����ӷ����¶ȹ����ܽ�����С��(3)NOx����ԭ�������������غ�͵��ӵ�ʧд������ʽ�� 4NOx+��5��2x��O2+2H2O= 4HNO3������ҺM��ͨ��Cl2��FeCl2������FeCl3,��ѭ��ʹ�ã�ѡb�� �𰸣� 4NOx+��5��2x��O2+2H2O= 4HNO3��b��(4)Fe3����3H2O Fe(OH)3��3H�� ,����CuO,ʹˮ��ƽ��������У�������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���������ȹ�����Cu2�� ��ˮ�⣬������������Cu2+��ˮ�⣨�������������ʣ�����:������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���� ����Cu2+��ˮ�⣨�������������ʣ�
Fe(OH)3��3H�� ,����CuO,ʹˮ��ƽ��������У�������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���������ȹ�����Cu2�� ��ˮ�⣬������������Cu2+��ˮ�⣨�������������ʣ�����:������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���� ����Cu2+��ˮ�⣨�������������ʣ�
 Fe(OH)3��3H�� ,����CuO,ʹˮ��ƽ��������У�������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���������ȹ�����Cu2�� ��ˮ�⣬������������Cu2+��ˮ�⣨�������������ʣ�����:������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���� ����Cu2+��ˮ�⣨�������������ʣ�
Fe(OH)3��3H�� ,����CuO,ʹˮ��ƽ��������У�������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���������ȹ�����Cu2�� ��ˮ�⣬������������Cu2+��ˮ�⣨�������������ʣ�����:������Һ��pH��ʹ��Ԫ�أ�Fe3+����ȫת��ΪFe(OH)3���� ����Cu2+��ˮ�⣨�������������ʣ�
��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
�����Ŀ