��Ŀ����
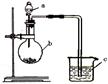
����ѧϰС������ͼװ�ã��̶�װ�ü���Ƥ����ȥ��̽���Ҷ��ᣨHOOC��COOH�����ȷֽ�IJ��ֲ����֪����ʱCO+PdCl2+H2O==CO2��+Pd��(��)+2HCl
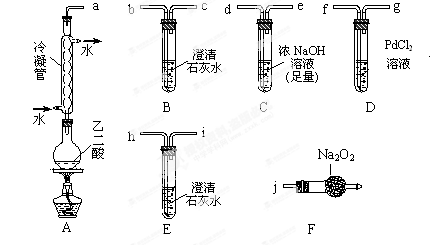
��1�����飺
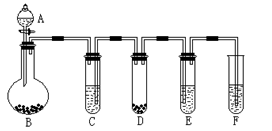
�ٰ��ӿ�˳��a-b-c-d-e-f-g-h����װ�ý���ʵ�顣B����Һ����ǣ�֤���ֽ������ �� E����Һ����ǣ�֤���ֽ������ ��
���Ҷ������ȷֽ�Ļ�ѧ����ʽΪ ��
��2�����飺
�ٽ��ӿ�a��j���ӽ���ʵ�飬�۲쵽F�����ɵ������ʹ�����ǵ�ľ����ȼ����F������Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
��ȥ��������,���ӿ�a��b���ӽ���ʵ��, B����Һ�����,������˵�����������һ�ַֽ����,����Ϊ ��

��1�����飺
�ٰ��ӿ�˳��a-b-c-d-e-f-g-h����װ�ý���ʵ�顣B����Һ����ǣ�֤���ֽ������ �� E����Һ����ǣ�֤���ֽ������ ��
���Ҷ������ȷֽ�Ļ�ѧ����ʽΪ ��
��2�����飺
�ٽ��ӿ�a��j���ӽ���ʵ�飬�۲쵽F�����ɵ������ʹ�����ǵ�ľ����ȼ����F������Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
��ȥ��������,���ӿ�a��b���ӽ���ʵ��, B����Һ�����,������˵�����������һ�ַֽ����,����Ϊ ��
�� 8�֣���1����CO2�� CO�� ��HOOC��COOH CO2����CO����H2O
CO2����CO����H2O
��2����2Na2O2��2CO2��2Na2CO3��O2 �ڲ�����ʯ��ˮ��������
 CO2����CO����H2O
CO2����CO����H2O ��2����2Na2O2��2CO2��2Na2CO3��O2 �ڲ�����ʯ��ˮ��������
�����������1����B�г���ʯ��ˮ����ǣ�֤���ֽ������CO2������װ��C�������dz�ֳ�ȥCO2����ֹ�Ժ��ʵ��������ĸ��ţ�E�г���ʯ��ˮ����ǣ�˵����CO2�������Ҷ��������CO2��Cװ�����Ѿ���NaOH������ȫ��CO2�IJ�����Դ��Dװ���и�����ض�CO��������D������Ϊ��Һ��ɫ�����dz����֤���ֽ������CO��
���Ҷ������ȷֽ�Ļ�ѧ����ʽΪHOOC��COOH
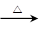 CO2��+CO��+H2O��
CO2��+CO��+H2O����2���ٽӿ�a��j���ӽ���ʵ�飬�۲쵽F�����ɵ������ʹ�����ǵ�ľ����ȼ��˵��������������ͨ�������ܺ������Ҫ��CO2��CO2��Na2O2��Ӧ�Ļ�ѧ����ʽΪ��2CO2+Na2O2��2Na2CO3+O2��
�����ڲ����ӷ�����ȥ��������,���ӿ�a��b���ӽ���ʵ�飬�����»ӷ����IJ����ʯ��ˮ��Ӧ���������ԵIJ���ƶ�����ǣ����Բ���˵����
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣����ʱע��������⣬�ر������ʼ���ʱҪ�ų��������ʵ����ʸ��ţ��Եó���ȷ���ۡ����������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
�����Ŀ
 ����Һ
����Һ
 ��Һ
��Һ
 ZnO
ZnO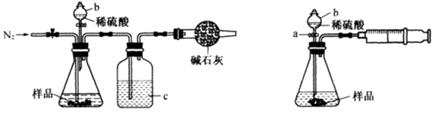
 װ��D�У�
װ��D�У�  װ��F�У�
װ��F�У�