��Ŀ����
����Ŀ����Ȼ������Ҫ�ɷ��Ǽ��飬����һ����Ҫ�Ļ���ԭ�ϡ�
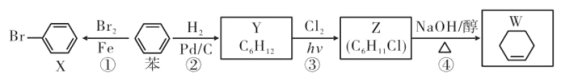
��1��CH4��CO2������Ӧ���Ƶúϳ�����CH4��g��+CO2��g��=2CO��g��+2H2��g�� ��H
��֪����Ӧ1��CH4��g��=C��s��+2H2��g�� ��H1=+75kJ/mol
��Ӧ2: 2CO��g��=C��s��+CO2��g�� ��H2=-172kJ/mol ��ô���Ӧ����H=__________kJ/mol��
��2����ҵ�Ͽ���CO��H2�ϳɶ����ѣ��䷴ӦΪ: 2CO��g��+4H2 ��g��=CH3OCH3 ��g��+H2O��g�� ��H=-204.7 kJ/mol ��ʼ������ͬʱ���ֱ���A�����£���B�����ȣ����������ڷ�Ӧ����Ӧ��ʼʱ�������ķ�Ӧ����A_______B������>������<������=������ͬ����ƽ��ʱCO��ת����A_______B��
��3����ҵ�Ͽ���CO��H2�ϳɼ״����䷴ӦΪCO��g��+2H2��g��=CH3OH��g�� ��H=-90.1kJ��md���¶�Tʱ�����ݻ�Ϊ2L��ij�ܱ������н���������Ӧ����Ӧ���������������ͼ��ʾ��
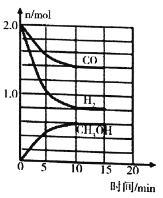
�������ܱ����÷�Ӧ�Ѵﵽƽ��״̬����__________��
a v��H2��=2v��CH3OH�� b c�� CO��/c�� CH3OH��=7: 3ʱ
c c��H2��/c�� CH3OH��=2: 1ʱ d �����������ѹǿ���ٱ仯
�ڸû�ѧ��Ӧ10min������CH3OH�ķ�Ӧ����v��CH3OH��= __________��
�۸��¶��£���Ӧ��ƽ�ⳣ��Ϊ__________ ���г�����ʽ���ɣ� ��
����������������ʱ,15mimʱ���������м���2 mol CO�� x mol H2��ƽ��ʱCO��ת������ԭƽ����ͬ����x=__________��
���𰸡�+247 = > bd 0.03mol��L-1��min-1 ![]() 1.2
1.2
��������
��1�����ݸ�˹���ɿ�֪����Ӧ1-��Ӧ2��CH4��g��+CO2��g��=2CO��g��+2H2��g����
��2����Ӧ��ʼʱ���������з�Ӧ���Ũ����ͬ���¶ȵ���ͬ���������ķ�Ӧ����A=B����Ӧ���ȡ�B���¶ȸߣ�ƽ�������ƶ���ƽ��ʱCO��ת����A>B��
��3���ٿ��淴Ӧ����ƽ��ʱ��ͬ�����ʵ�������������ұ��ֲ��䣬����ֵ�Ũ�ȡ��������ֲ��䣬�ɴ�����������һЩ�����䣬�ж�ƽ���������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����ٱ仯˵������ƽ�⣻
�ڸ���v=![]() ���㣻
���㣻
�۸÷�Ӧ��ƽ�ⳣ��K=![]() ��
��
���г�����ʽ������ƽ�ⳣ��������㡣
��1����֪����Ӧ1��CH4��g��=C��s��+2H2��g�� ��H1=+75kJ/mol
��Ӧ2: 2CO��g��=C��s��+CO2��g�� ��H2=-172kJ/mol
���ݸ�˹���ɿ�֪����Ӧ1-��Ӧ2��CH4��g��+CO2��g��=2CO��g��+2H2��g�� ��H=��H1-��H2=+75kJ��mol��1-��-172kJ��mol��1��=+247kJ/mol��
�ʴ�Ϊ����H=+247kJ/mol��
��2��2CO��g��+4H2 ��g��=CH3OCH3 ��g��+H2O��g�� ��H=-204.7 kJ/mol �Ƿ��ȷ�Ӧ����ʼ������ͬʱ���ֱ���A�����£���B�����ȣ����������ڷ�Ӧ����Ӧ��ʼʱ���������з�Ӧ���Ũ����ͬ���¶ȵ���ͬ���������ķ�Ӧ����A=B����Ӧ���ȡ�B���¶ȸߣ�ƽ�������ƶ���ƽ��ʱCO��ת����A>B��
�ʴ�Ϊ��=��>;
��3����a. v��H2��=2v��CH3OH������˵�����淴Ӧ���ʹ�ϵ����a��ѡ��
b.��ͼ c�� CO����c�� CH3OH��=1.4:0.6=7: 3ʱ����ƽ��ʱŨ�ȱȣ���bѡ��
c .��ͼc��H2����c�� CH3OH��=0.8:0.6==4: 3ʱ����ƽ��ʱŨ�ȱȣ���c��ѡ��
d. CO��g��+2H2��g��=CH3OH��g�������������С�ķ�Ӧ�������������ѹǿ���ٱ仯��˵����Ӧ�ﵽƽ��״̬����dѡ��
��ѡbd��
�ڸû�ѧ��Ӧ10min������CH3OH�ķ�Ӧ����v��CH3OH��= ![]() =0.03mol��L-1��min-1��
=0.03mol��L-1��min-1��
����ͼCO��H2��CH3OH��Ũ�ȷֱ��ǣ�0.3��0.7��0.43mol��L-1�����¶��£��÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() ��
��
����������������ʱ,15mimʱ���������м���2 mol CO�� x mol H2��ƽ��ʱCO��ת������ԭƽ����ͬ��
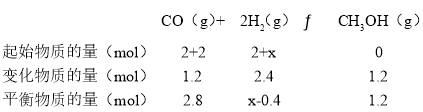
���¶��£��÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() =
=
��x=1.2��

����Ŀ��
��. 1����������л�����з��࣬����ȷ�𰸣���ţ���д�ڱ����Ӧ�����
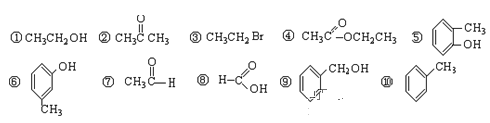
������ | ±���� | �� | �� | ȩ | ͪ | ���� | �� |
______ | ______ | ______ | ______ | ______ | ______ | ______ | ______ |
2������10�������У���Ϊλ���칹����ǣ���д��ţ�______________________��
��.��ϵͳ������д�������������ƣ�
1��(CH3)2CHCH=CHCH(C2H5)CH2CH3 ______________________
2��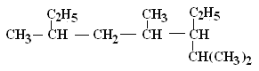 ______________________
______________________
��. ����A��ϵͳ����Ϊ��1-��ϩ������ṹ��ʽΪ____����д����û����ﺬ����ͬ�����ŵ���������ͬ���칹��Ľṹ��ʽ����Ӧ���ơ�_________________________
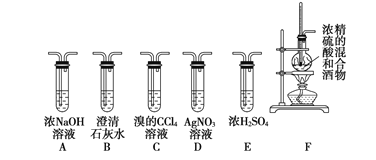
����Ŀ��������Ȼ������Ƶ��������ƣ�Na2NO2����������Ӧ��ʮ�ֹ㷺��������������ֲĻ�ʴ��������Ʒ��ɫ���ȡ���֪��2NO+Na2O2=2NaNO2 2NO2+Na2O2=2NaNO3
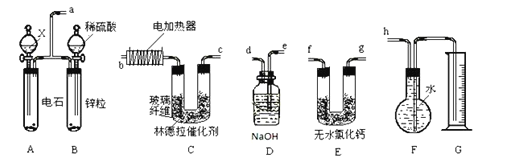
I��ijѧϰС�������ͼװ���Ʊ��������ƣ��г�װ����ʡ�ԣ�
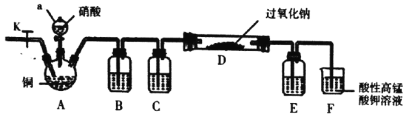
��1������a������Ϊ________��
��2��װ��B��C��E�е����ƿ�ѡ��___��
A ˮ��Ũ���ᡢŨ����
B ����������Һ��Ũ���ᡢŨ����
C ���Ը��������Һ��Ũ���ᡢ����������Һ
D ˮ��Ũ���ᡢ����������Һ
��3����Ӧ��ʼʱ�ȴ�ֹˮ��K��ͨ�뵪����F�в����������ݣ��ò�����Ŀ����________��
��4��װ��D�е�ʵ��������________��
��5��װ��F�з�����Ӧ�����ӷ���ʽΪ________��
��ҵ�Ͽ������ռ���Һ�������᳧��β���Ƶ��������ơ�ijѧϰС���������ʵ�鷽���ⶨ�ò�Ʒ���������Ƶ�����������ȡ1.500g��Ʒ���250mL��Һ��ȡ25.00mL��Һ����ƿ�м��������ϡ����͵⻯����Һ��ַ�Ӧ�����ʵ���ָʾ������0.1000mol/L Na2S2O3����Һ���еζ�����¼�������±���
ʵ����� | 1 | 2 | 3 |
Na2S2O3����Һ���/mL | 20.02 | 19.98 | 20.00 |
��֪�� 2NaNO2+2KI+2H2SO4 =2NO��+I2+2H2O+Na2SO4+K2SO4 2Na2S2O3+I2=Na2S4O6+2NaI
��6�����ʵ�ָʾ����________
��7���ò�Ʒ�Ĵ���Ϊ________��
��8����������,ͬѧ��Ϊ�ò�Ʒ�п��ܺ��������ƣ�����ʹ�ⶨ���________������ƫ��������ƫ����������Ӱ������ ��
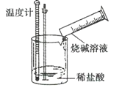
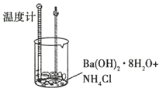
����Ŀ��ijʵ��̽��С���������ʵ�飬̽����ѧ��Ӧ�е������仯��
ʵ��� | ʵ��� |
|
|
�¶ȼƶ������� | �¶ȼƶ������� |
�����ж�һ����ȷ���ǣ� ��
A.ʵ��ٵķ�Ӧ�л�ѧ���������յ���������ʵ���
B.ʵ��ٵķ�Ӧ�л�ѧ���������յ�����С��ʵ��ڵķ�Ӧ�л�ѧ���γɷų�������
C.ʵ���˵���кͷ�Ӧ�ų�����
D.ʵ���˵�������а������ɵķ�Ӧ����������