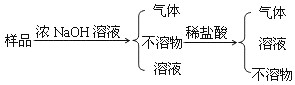
��Ŀ����
ij����Al��Al2O3��ĩ��ɵĻ������Ʒ��������ͼװ�òⶨ�������Al��������������֪��Ʒ����Ϊ2.58g����ƿ����NaOH��Һ��������Ϊ185.72g����ÿ����ͬʱ����õ�����ƽ�����������
��1��Al��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ______��
��2����Ӧ�в�������������Ϊ______g
��3������Ʒ��Al������������______��Ҫ���м�����̣���

| �������� | ������g�� | |
| ��ƿ + NaOH��Һ + ���� | ��1�� | 188.30 |
| ��2�� | 188.28 | |
| ��3�� | 188.25 | |
| ��4�� | 188.24 | |
| ��5�� | 188.24 |
��2����Ӧ�в�������������Ϊ______g
��3������Ʒ��Al������������______��Ҫ���м�����̣���

��1�������������Ʒ�Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2����������������Ҫ�����ݣ�����ͼ�����ݿ�֪�����غ��ص�����Ϊ188.24g�����Է�Ӧ��������������Ϊ188.30-188.24=0.06g
��3�����ݷ�Ӧ��֪��2Al+2NaOH+2H2O=2NaAlO2+3H2������������0.03mol����Ҫ�����ʵ���Ϊ0.02mol��
��Ʒ��Al������Ϊ0.02mol��27=0.541g������Ʒ��Al����������Ϊ
��100%=20.9%��
�ʴ�Ϊ����������0.03mol����Ҫ�����ʵ���Ϊ0.02mol����Ʒ��Al������Ϊ0.02mol��27=0.541g������Ʒ��Al����������Ϊ
��100%=20.9%��
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2����������������Ҫ�����ݣ�����ͼ�����ݿ�֪�����غ��ص�����Ϊ188.24g�����Է�Ӧ��������������Ϊ188.30-188.24=0.06g
��3�����ݷ�Ӧ��֪��2Al+2NaOH+2H2O=2NaAlO2+3H2������������0.03mol����Ҫ�����ʵ���Ϊ0.02mol��
��Ʒ��Al������Ϊ0.02mol��27=0.541g������Ʒ��Al����������Ϊ
| 0.54g |
| 2.58g |
�ʴ�Ϊ����������0.03mol����Ҫ�����ʵ���Ϊ0.02mol����Ʒ��Al������Ϊ0.02mol��27=0.541g������Ʒ��Al����������Ϊ
| 0.54g |
| 2.58g |

��ϰ��ϵ�д�
�����Ŀ