��Ŀ����
�����й�ʵ��ı����У�������ǣ� ��
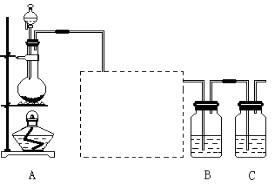
| A����ȥ�Ҵ���ˮ����������ʯ�ң������ռ������ |
| B��������۵�ˮ������������������ˮ��Һ��ֱ�Ӽ�������Cu(OH)2��Һ��Ȼ����ȣ��۲��Ƿ��к�ɫ�������� |
| C����ȥ���������е�����ӱ���̼������Һ���������Һ����ˮ�� |
| D��������Һ�����ƣ��ڽྻ���Թ��м�2% AgNO3��Һ1��2 mL����μ���2%ϡ��ˮ���ߵα���������ǡ���ܽ�ʱΪֹ |
B
���������A����ʯ�������Ƽ�����ˮ�����������ƣ���˳�ȥ�Ҵ���ˮ����������ʯ�ң������ռ�����T�ɣ�A��ȷ��B������ˮ�������������½��У���ȩ��������������ͭ����Һ�ķ�Ӧ���ڼ��������½��еģ���B����ȷ��C������̼������Һ���Խ��������������ܽ�ȣ��������ᣬ���Գ�ȥ���������е�����ӱ���̼������Һ���������Һ����ˮ�㣬C��ȷ��D���ڽྻ���Թ��м�2% AgNO3��Һ1��2 mL����μ���2%ϡ��ˮ���ߵα���������ǡ���ܽ�ʱΪֹ����ʱ������Һ��������Һ��D��ȷ����ѡB��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 H2O + CH3CH2��O��CH2CH3 (����)
H2O + CH3CH2��O��CH2CH3 (����)


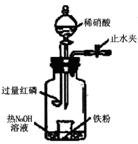

 ��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ ��