��Ŀ����
A��B��C��DΪ���ֶ�����Ԫ�أ�Aԭ�Ӻ������1�����ӣ�B��CԪ�ص��������+4��+5��DԪ�س���-2�ۣ���֪B��C��D��ԭ�Ӵ�����������Ϊ2��
��1����Ҫ��д����AԪ�ط���____________��Bԭ�ӽṹʾ��ͼ____________��C���ʷ��ӵĵ���ʽ
____________��
��2��X��Y��Z��W���ֻ����������������Ԫ�أ��Ҷ������Σ�����X��Y��Z���ֻ��������ʽ�ж�ֻ��һ��Bԭ�ӣ�X��Y�������Ӵ�һ����λ��ɣ���X�������ӱ�Y��������һ��Dԭ�ӣ�Z��W�������Ӷ���������λ�ĸ���ɣ�W�к�������Bԭ�ӡ�
��д��Z��W�Ļ�ѧʽ��ZΪ____________��WΪ____________��
��д��Y����Һ�����NaOH��Ӧ�����ӷ���ʽ________________________��
��1����Ҫ��д����AԪ�ط���____________��Bԭ�ӽṹʾ��ͼ____________��C���ʷ��ӵĵ���ʽ
____________��
��2��X��Y��Z��W���ֻ����������������Ԫ�أ��Ҷ������Σ�����X��Y��Z���ֻ��������ʽ�ж�ֻ��һ��Bԭ�ӣ�X��Y�������Ӵ�һ����λ��ɣ���X�������ӱ�Y��������һ��Dԭ�ӣ�Z��W�������Ӷ���������λ�ĸ���ɣ�W�к�������Bԭ�ӡ�
��д��Z��W�Ļ�ѧʽ��ZΪ____________��WΪ____________��
��д��Y����Һ�����NaOH��Ӧ�����ӷ���ʽ________________________��
��1��H�� ��
��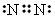
��2����(NH4)2CO3��(NH4)2C2O2����NH4++HCO3-+2OH-==NH3��H2O+2H2O+CO32-
 ��
��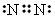
��2����(NH4)2CO3��(NH4)2C2O2����NH4++HCO3-+2OH-==NH3��H2O+2H2O+CO32-

��ϰ��ϵ�д�
�����Ŀ

