��Ŀ����
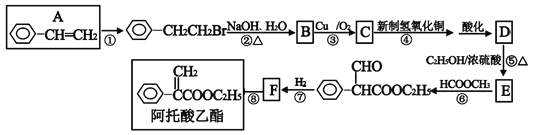
����Ŀ�����������������ڸ�������θ�������μ���������������һ�ֺϳ�·������ͼ��ʾ��
��ش��������⣺
��1��A��������__________���������������������ŵ�����____________��
��2����Ӧ�ķ�Ӧ����Ϊ______________��F�Ľṹ��ʽ____________��
��3����Ӧ�۵ķ���ʽ______________________________________����Ӧ�ݵķ���ʽ______________________________________��
��4���йذ�����������˵����ȷ����____________��
A��1mol�����������������4molH2�ӳ�
B����ʹ���Ը��������ɫ��Ҳ��ʹ��ˮ��ɫ
C���ܷ����ӳɡ��Ӿۡ�������ˮ��ȷ�Ӧ
D������ʽΪC11H13O2
��5��D��ͬ���칹���ж��֣����Ϻ��б���������̼�����Ʒ�Ӧ�ų������ͬ���칹����_________�֣�������D����
���𰸡� ����ϩ ������̼̼˫�� ȡ����Ӧ ![]() 2
2![]() +O2
+O2![]() 2
2![]() +2H2O
+2H2O ![]() ABC 3
ABC 3
�������������и����ʵ�ת����ϵ��֪��A���廯�ⷢ���ӳɷ�Ӧ����![]() ��
��![]() �ڼ���������ˮ�⣬����ȡ����Ӧ����B��BΪ
�ڼ���������ˮ�⣬����ȡ����Ӧ����B��BΪ![]() ��B����������Ӧ����C��CΪ
��B����������Ӧ����C��CΪ![]() ��C�ٱ��������ữ���D��DΪ
��C�ٱ��������ữ���D��DΪ![]() ��D���Ҵ�����ȡ��(����)��Ӧ����E��EΪ
��D���Ҵ�����ȡ��(����)��Ӧ����E��EΪ![]() ��E������������ȡ����Ӧ����
��E������������ȡ����Ӧ����![]() ��
��![]() �����������ӳɷ�Ӧ����FΪ
�����������ӳɷ�Ӧ����FΪ![]() ��F�ٷ�����ȥ��Ӧ�ð�����������
��F�ٷ�����ȥ��Ӧ�ð�����������
(1)AΪ![]() ������Ϊ����ϩ������������������������������̼̼˫�����ʴ�Ϊ������ϩ��������̼̼˫����
������Ϊ����ϩ������������������������������̼̼˫�����ʴ�Ϊ������ϩ��������̼̼˫����
(2)����������������Ӧ����![]() ������������ȡ����Ӧ����
������������ȡ����Ӧ����![]() ��F�Ľṹ��ʽΪ
��F�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��ȡ����Ӧ��
���ʴ�Ϊ��ȡ����Ӧ��![]() ��
��
(3)��Ӧ�۵ķ���ʽΪ![]() ����Ӧ�ݵķ���ʽΪ
����Ӧ�ݵķ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
(4)��������������������̼̼˫���������Ƚṹ��A��1ml�����������������4molH2�ӳɣ�A��ȷ��B������̼̼˫������ʹ���������ɫҲ��ʹ��ˮ��ɫ��B��ȷ��C������̼̼˫�����ܷ����ӳɡ��Ӿۡ�������Ӧ�������������ܷ���ˮ�ⷴӦ��C��ȷ��D������ʽΪC11H12O2��D���ʴ�Ϊ��ABC��
(5)D��ͬ���칹���ж��֣����Ϻ��б���������̼�����Ʒ�Ӧ�ų����壬�����Ȼ���ͬ���칹��Ϊ����������-CH3��-COOH���ֱ�Ϊ![]() ��
��![]() ��
��![]() �����Թ���3��(������D)���ʴ�Ϊ��3��
�����Թ���3��(������D)���ʴ�Ϊ��3��

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�����Ŀ��ijѧϰС��ͨ��ʵ���о�Na2O2��ˮ�ķ�Ӧ��
���� | ���� |
��ʢ��4.0g Na2O2���ձ��м���50mL����ˮ | ���ҷ�Ӧ��������ʹ������ľ����ȼ�����壬�õ�����ɫ��Һa |
����Һa�е������η�̪ | ������Һ��� ����10���Ӻ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
��1��Na2O2�ĵ���ʽΪ___________������ˮ��Ӧ�����ӷ���ʽ��__________________��
��2����ͬѧ��Ϊ������Һ��ɫ����Һa�д��ڽ϶��H2O2��H2O2���̪�����˷�Ӧ����ʵ��֤ʵ��H2O2�Ĵ��ڣ�ȡ������Һa�������Լ�___________���ѧʽ���������������
��3����ͬѧ�������ϻ�Ϥ����KMnO4��������H2O2���ⶨ�京����ȡ20.00mL��Һ����ϡH2SO4�ữ����0.002mol/L KMnO4��Һ�ζ����������壬��Һ��ɫ�����յ�ʱ������10.00mL KMnO4��Һ��
��ʵ���У��ζ�ʱKMnO4��ҺӦװ��__________�����ʽ�ζ����С�
��������������÷�Ӧ����ת�Ƶķ������Ŀ��_______________��
2MnO4-+5H2O2+6H+ = 2Mn2++5O2��+8H2O��
����Һa�� c(H2O2)=___________ mol/L��