��Ŀ����
��֪�ס��ҡ�����Ϊ�������壬���м��ڿ����к�����࣬������ͬ�������ܶ���С�����д̼�����ζ����һ�����������ĸ�ԭ�ӹ��ɣ�
��1��ʵ�����п�����ͼA��Bװ������Ӧ��ҩƷ�Ƶñ���
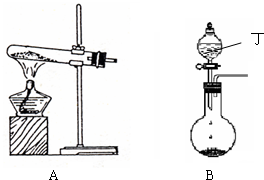
��A���Թ��ڷ�Ӧ�Ļ�ѧ����ʽ��______��
��B�з�Һ©����ʢ�ŵ����ʶ���______��Բ����ƿ�ڵ�������______�����������ƣ�
��2����ҵ�Ͻ������ڸ��¡���ѹ����������������ȡ������ͼ�Ǽ��ҷ�Ӧ�����������仯ͼ��
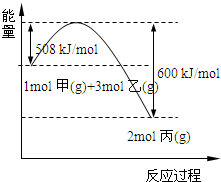
�÷�Ӧ���Ȼ�ѧ����ʽ��______��
��3������������ȼ�յķ�Ӧ���û���Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��______��
��4���ٽ�����CO2����ͨ�뱥��ʳ��ˮ����̼�����ƾ�����������Ӧ�����ӷ���ʽ��______��
��Ϊ����֤��������Ȳ���NH4HCO3��Ҳ����NaCl����NaHCO3�����ʵ�鷽�����£�������в���ʵ�鱨�棺
��5�����ã�1���е�A��ȡ������������ƿͨ���������ռ�������״������Ȼ�������Ȫʵ�飮��ˮ���뵽��ƿ�����3/5ʱ��Һ�治����������ʱ�����ر�ֹˮ�У���ƿ����Һ�����ʵ����ʵ���Ũ����______ mol/L����ȷ��0.001����
��1��ʵ�����п�����ͼA��Bװ������Ӧ��ҩƷ�Ƶñ���

��A���Թ��ڷ�Ӧ�Ļ�ѧ����ʽ��______��
��B�з�Һ©����ʢ�ŵ����ʶ���______��Բ����ƿ�ڵ�������______�����������ƣ�
��2����ҵ�Ͻ������ڸ��¡���ѹ����������������ȡ������ͼ�Ǽ��ҷ�Ӧ�����������仯ͼ��

�÷�Ӧ���Ȼ�ѧ����ʽ��______��
��3������������ȼ�յķ�Ӧ���û���Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��______��
��4���ٽ�����CO2����ͨ�뱥��ʳ��ˮ����̼�����ƾ�����������Ӧ�����ӷ���ʽ��______��
��Ϊ����֤��������Ȳ���NH4HCO3��Ҳ����NaCl����NaHCO3�����ʵ�鷽�����£�������в���ʵ�鱨�棺
| ʵ����� | ʵ������ | ���� | ��Ӧ�����ӷ���ʽ |
| ȡ�����������Թ��У���ּ��� | �Թ����й���ʣ�� | ______ | ______ |
| ______ | ����ȫ���ܽ⣬�����ݲ��� | ______ | ______ |
��1����ʵ�����ù����Ȼ�狀��������Ƽ�����ȡ��������Ӧ����ʽΪ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
��ʵ���һ�������Ũ��ˮ�������ƻ��������ơ���ʯ����ȡ����������Һ����Ũ��ˮ�������������ƻ��������ƻ��ʯ�ң��ʴ�Ϊ��Ũ��ˮ�������ƣ��������ƻ��ʯ�ң���
��2����H=-��600-508��=-92kJ/mol�����Ը÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92kJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92kJ/mol��
��3����ȼ�����£�������������Ӧ���ɵ�����ˮ����Ӧ����ʽΪ��4NH3+3O2
2N2+6H2O���ʴ�Ϊ��4NH3+3O2
2N2+6H2O��
��4���ٰ�����ˮ��Ӧ���ɰ�ˮ����ˮ����������̼��Ӧ����̼����泥�̼�����Ƶ��ܽ��С��̼����泥���ԭ����Һ�DZ�����Һ�����Ի�����̼�����ƣ����ӷ���ʽΪ��NH3+CO2+H2O+Na+=NaHCO3��+NH4+���ʴ�Ϊ��NH3+CO2+H2O+Na+=NaHCO3��+NH4+��
�ڽ�������Ⱥ��й���ʣ�࣬˵���ù��岻��̼����泥�����ȴ����Թ��м����������ᣬ���������ɣ�˵���ù���Ϊ̼���ζ������Ȼ��ƣ���Ӧ����ʽΪ��CO32-+2H+=H2O+CO2����
�ʴ�Ϊ��
��5��c=
=
=
=
mol/L=0.0045mol/L���ʴ�Ϊ��0.0045��
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��ʵ���һ�������Ũ��ˮ�������ƻ��������ơ���ʯ����ȡ����������Һ����Ũ��ˮ�������������ƻ��������ƻ��ʯ�ң��ʴ�Ϊ��Ũ��ˮ�������ƣ��������ƻ��ʯ�ң���
��2����H=-��600-508��=-92kJ/mol�����Ը÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92kJ/mol��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92kJ/mol��
��3����ȼ�����£�������������Ӧ���ɵ�����ˮ����Ӧ����ʽΪ��4NH3+3O2
| ||
| ||
��4���ٰ�����ˮ��Ӧ���ɰ�ˮ����ˮ����������̼��Ӧ����̼����泥�̼�����Ƶ��ܽ��С��̼����泥���ԭ����Һ�DZ�����Һ�����Ի�����̼�����ƣ����ӷ���ʽΪ��NH3+CO2+H2O+Na+=NaHCO3��+NH4+���ʴ�Ϊ��NH3+CO2+H2O+Na+=NaHCO3��+NH4+��
�ڽ�������Ⱥ��й���ʣ�࣬˵���ù��岻��̼����泥�����ȴ����Թ��м����������ᣬ���������ɣ�˵���ù���Ϊ̼���ζ������Ȼ��ƣ���Ӧ����ʽΪ��CO32-+2H+=H2O+CO2����
�ʴ�Ϊ��
| ʵ����� | ʵ������ | ���� | ��Ӧ�����ӷ���ʽ |
| �þ��岻��NH4HCO3 | |||
| ����ȴ���Թ��м����������� | �þ��岻��NaCl����NaHCO3 | CO32-+2H+=H2O+CO2�� |
| n |
| V |
| ||
| V |
| 1 |
| Vm |
| 1 |
| 22.4 |

��ϰ��ϵ�д�
�����Ŀ
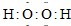
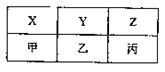
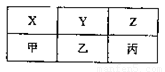
 ��4��X��Y��Z���ס��ҡ�������A��B��C�ֱ���D��
��4��X��Y��Z���ס��ҡ�������A��B��C�ֱ���D��