��Ŀ����
����Ŀ�������ͭ(��)���[KaCub(C2O4)c��xH2O]��һ����Ҫ�Ļ���ԭ�ϡ�
��1���������ͭ(��)��ؾ��������CuSO4�����K2C2O4��Һ��Ӧ�õ���������ͭ��Һ�л������ͭ�����ʵ�鲽��Ϊ�����������Ҵ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����������Ũ���ij�ʼ�λ������������������Ŀ����_____________________��
��2��ijͬѧΪ�ⶨ�����ͭ(��)��ص���ɣ���������ʵ�飺
�������ⶨCu2+��ȷ��ȡ0.7080 g��Ʒ����20.00 mL NH4ClNH3��H2O������Һ�ܽ⣬����ָʾ������0.1000 mol��L1��EDTA(Na2H2Y)����Һ�ζ����յ㣨���ӷ���ʽΪCu2++H2Y2![]() CuY2+2H+��������EDTA����Һ20.00 mL��
CuY2+2H+��������EDTA����Һ20.00 mL��
�������ⶨC2O42-��ȷ��ȡ0.7080 g��Ʒ����6.00 mLŨ��ˮ�ܽ⣬����30.00 mL 4.0 mol��L1�����ᣬϡ����100 mL��ˮԡ������70~80����������0.1000 mol��L1 KMnO4��Һ�ζ����յ㣬����KMnO4��Һ16.00 mL��
����֪����������MnO4-����ԭΪMn2+��������������Ӧ�����ӷ���ʽΪ___________��
���������ζ��յ��������______________________��
��ͨ������ȷ�������ͭ(��)��صĻ�ѧʽ��д��������̣���____________
���𰸡������Ҵ� 2![]() +5
+5![]() +16H+
+16H+![]() 2Mn2++10CO2��+8H2O ���������һ��KMnO4��Һʱ����Һ���dz��ɫ���Ұ���Ӳ���ɫ �ɲ������ķ�Ӧ���ӷ���ʽ��Cu2++H2Y2
2Mn2++10CO2��+8H2O ���������һ��KMnO4��Һʱ����Һ���dz��ɫ���Ұ���Ӳ���ɫ �ɲ������ķ�Ӧ���ӷ���ʽ��Cu2++H2Y2![]() CuY2+2H+���ɵù�ϵʽ��Cu2+��H2Y2���������У�
CuY2+2H+���ɵù�ϵʽ��Cu2+��H2Y2���������У�
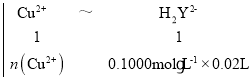
��ã�n(Cu2+)=0.002mol
�ɲ����������ӷ�Ӧ����ʽ��2MnO4-+5C2O42-+16H+![]() 2Mn2++10CO2��+8H2O���ɵù�ϵʽ��2MnO4-��5C2O42-�����У�
2Mn2++10CO2��+8H2O���ɵù�ϵʽ��2MnO4-��5C2O42-�����У�
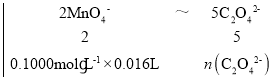
��ã�n(C2O42-)=0.004mol
���ݵ���غ㣬��֪n(K+)=0.004mol
���������غ�ԭ����![]() ��n(H2O)=
��n(H2O)=![]() ��
��

�ʲ����ͭ(��)��صĻ�ѧʽΪ��K2Cu(C2O4)2��2H2O��
��������
��1����������������Ϣ������ͭ�������Ҵ���ʹ���Ҵ����Խ�������ͭ���ܽ�ȣ������ھ���������������Ҵ����Ҵ��ӷ����ʿ�ͨ������ķ������ա�
��2���������в������ⶨCu2+��ԭ�������ĵ�EDTA�������ɼ����0.7080 g��Ʒ��Cu2+���������ݲ������ⶨC2O42-�Ĺ����У�KMnO4��C2O42-�����������·���������ԭ��Ӧ�����������ԭ��Ӧԭ�����ɼ����0.7080 g��Ʒ��C2O42-��������ϵ���غ�������غ㣬�ֱ�����K+��H2O������������ȷ�������ͭ(��)��صĻ�ѧʽ��
��1���Ҵ��ӷ����ʿ�ͨ������ķ������ա���Ϊ�������Ҵ���
��2��������������ԭ��Ӧԭ������֪���ӷ�Ӧ����ʽΪ��2MnO4-+5C2O42-+16H+![]() 2Mn2++10CO2��+8H2O����Ϊ��2MnO4-+5C2O42-+16H+
2Mn2++10CO2��+8H2O������2MnO4-+5C2O42-+16H+![]() 2Mn2++10CO2��+8H2O��
2Mn2++10CO2��+8H2O��
��KMnO4��ҺΪ�Ϻ�ɫ�����������һ��KMnO4��Һʱ����Һ���dz��ɫ���Ұ���Ӳ���ɫ��˵���ﵽ�ζ��յ㡣��Ϊ�����������һ��KMnO4��Һʱ����Һ���dz��ɫ���Ұ���Ӳ���ɫ��
���ɲ������ķ�Ӧ���ӷ���ʽ��Cu2++H2Y2![]() CuY2+2H+���ɵù�ϵʽ��Cu2+��H2Y2���������У�
CuY2+2H+���ɵù�ϵʽ��Cu2+��H2Y2���������У�
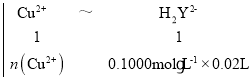
��ã�nspan>(Cu2+)=0.002mol
�ɲ����������ӷ�Ӧ����ʽ��2MnO4-+5C2O42-+16H+![]() 2Mn2++10CO2��+8H2O���ɵù�ϵʽ��2MnO4-��5C2O42-�����У�
2Mn2++10CO2��+8H2O���ɵù�ϵʽ��2MnO4-��5C2O42-�����У�
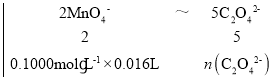
��ã�n(C2O42-)=0.004mol
���ݵ���غ㣬��֪n(K+)=0.004mol
���������غ�ԭ����![]() ��n(H2O)=
��n(H2O)=![]() ��
��

�ʲ����ͭ(��)��صĻ�ѧʽΪ��K2Cu(C2O4)2��2H2O��

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
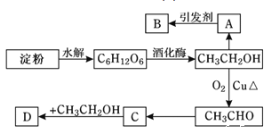
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�����Ŀ�������������Ϊ2 L�����ܱ������У���ӦCO(g)+2H2(g)![]() CH3OH(g) ��H��0�ﵽƽ�⣬�õ��������ݡ�����˵����ȷ����
CH3OH(g) ��H��0�ﵽƽ�⣬�õ��������ݡ�����˵����ȷ����
������� | �¶�/K | ���ʵ���ʼ���ʵ���/mol | ���ʵ�ƽ�����ʵ���/mol | ||
CO(g) | H2(g) | CH3OH(g) | CH3OH(g) | ||
�� | T1 | 0.40 | 0.80 | 0 | 0.24 |
�� | T2 | 0 | 0 | 0.40 | 0.20 |
�� | T2 | a | b | ||
A.��Ӧ�¶ȣ�T1��T2
B.��ƽ��ʱ��ת���ʣ���(CO,��)+��(CH3OH,��)��1
C.���������У���ƽ��ʱ��CO��ת���ʴ���H2����![]()
D.���������У�����ʼʱ����0.4 mol CO��0.4 mol H2��0.4 mol CH3OH�����ʱv(��)��v(��)
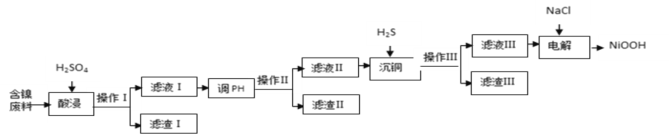
����Ŀ��NiOOH������ˮ��������ص��������ϡ�����ij�������ϣ���Ҫ�ɷ�ΪNiO��������SiO2��Fe2O3��CuO�������ʣ��Ʊ�NiOOH�Ĺ�ҵ������ͼ��
��֪��
Fe(OH)3 | Cu(OH)2 | Ni(OH)2 | |
��ʼ������pH | 1.5 | 4.4 | 6.7 |
������ȫ��pH | 3.7 | 6.9 | 9.2 |
(1)�������ٶȵķ���Ϊ_______________________��_____________________(��д2��)
(2)������ijɷ�Ϊ__________����������ΪFe(OH)3����pH���ķ�ΧΪ_____________.
(3)��ͭʱ������Ӧ�����ӷ���ʽΪ______________________________________________��
(4)�������ǿ����ڼ�����������������ClO-������ClO-��Ni2+�����Ĺ��̡���д�����������з�����Ӧ�����ӷ���ʽ____________________________________________________��