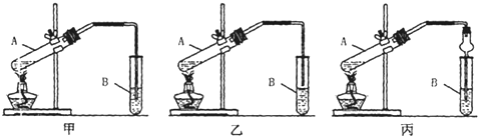
��Ŀ����
����Ŀ��(1)������ѧ��Ӧ���ʵ���Ҫ�����Dzμӷ�Ӧ�����ʵ�________��Ӱ�컯ѧ��Ӧ���ʵ�������_______________________(������2��)��
(2)H2C2O4��KMnO4��Ӧʱ����ת��ΪCO2��H2O��ʵ������________________
(3)������������Ba(OH)2��8H2O���Ȼ�立�Ӧ����ʽ_________________��
(4)Na2S2O3��ϡH2SO4��Ӧ���ӷ���ʽ_______________________��
(5)������KI��H2SO4�Ļ����Һ�м��������Һ������һ��ʱ��ʵ������________
(6)��H2O2��Һ�м�������MnO2ʵ������____________
(7)��1 g������ȫ�ֽ�Ϊ����������ʱ������2.72 kJ�����������Ȼ�ѧ����ʽ_______________
(8)�ɽ��ʯ(TiO2)��ȡ����Ti���漰�IJ���Ϊ��TiO2��TiCl4+Mg��Ti����֪��
��C(s)+O2(g)=CO2(g) ��H1=��393.5 kJ/mol
��2CO(g)+O2(g)=2CO2(g) ��H2=��566 kJ/mol
��TiO2(s)+2Cl2(g)=TiCl4(s)+O2(g) ��H3=+141 kJ/mol
��TiO2(s)+2Cl2(g)+2C(s)=TiCl4(s)+2CO(g)����H=__________
���𰸡��������� �¶ȡ�Ũ�ȡ�ѹǿ�������� �������ݣ�ͬʱ������Һ����ɫ��ɫ Ba(OH)28H2O+2NH4Cl=BaCl2+2NH3��+10H2O S2O32- +2H+=S��+SO2��+H2O ��Һ��Ϊ��ɫ �������� 2NH3(g)=N2(g)+3H2(g)����H=+92.4 8 kJ/mol -80 kJ/mol
��������
(1)��ѧ��Ӧ�ܷ��������п����̶��ɲμӷ�Ӧ�����ʱ������ʾ�������������ͬ������£�Ҳ���ܷ�Ӧ���¶ȡ���Ũ�ȡ�ѹǿ���������ܼ����������Ӱ�죻
(2)H2C2O4��KMnO4�ᷢ��������ԭ�����ݷ�Ӧ���������ʵ�״̬����ɫ�仯����ʵ������
(3)���߷������ֽⷴӦ���ݴ���д��Ӧ����ʽ��
(4) Na2S2O3��ϡH2SO4��Ӧ���������ơ�S��SO2��H2O���ݴ���д����ʽ��
(5)�����е���������Һ���ܽ⣬����KI����I2��I2�����ۣ���Һ��Ϊ��ɫ��
(6)MnO2��H2O2�ֽ�Ĵ�����
(7)�ȼ���1 g���������ʵ�����Ȼ���Ϸ�Ӧ�������������仯���Ӧ��д����Ӧ���Ȼ�ѧ����ʽ��
(8)���ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ���ӣ��Ϳ��Եõ���Ӧ��Ӧ���Ȼ�ѧ����ʽ��
(1)��ѧ��Ӧ�ܷ��������п����ɷ�Ӧ�ﱾ�����ʾ�����ͬʱҲ���¶ȡ�ѹǿ���������������Ӱ�죻
(2)H2C2O4���л�ԭ�ԣ���KMnO4���������ԣ����߷�Ӧʱ�����ᱻ��������CO2���壬������ر���ԭ������ɫ��Mn2+��ʹ��Һ��ɫ��dz������ɫ����˿�����ʵ�������������ݲ�������Һ����ɫ��ɫ��
(3)������������Ba(OH)2��8H2O���Ȼ�炙�Ϸ������ֽⷴӦ����BaCl2��NH3��H2O����Ӧ�Ļ�ѧ����ʽΪ��Ba(OH)28H2O+2NH4Cl=BaCl2+2NH3��+10H2O��
(4) Na2S2O3��ϡH2SO4��Ӧ���������ơ�S��SO2��H2O���������ʵ��ܽ��Լ�����ʵ�ǿ������֪�÷�Ӧ�����ӷ���ʽΪ��S2O32- +2H+=S��+SO2��+H2O��
(5)�����е������ں���KI��H2SO4�Ļ����Һ���ܽ⣬����KI����I2��I2�����ۣ���Һ��Ϊ��ɫ���ʿ�����ʵ�������ǣ���Һ��Ϊ��ɫ��
(6)H2O2���ȶ�����ֽ����H2O��O2��MnO2��H2O2�ֽ�Ĵ�������˻ῴ�������������ݣ�
(7)1 g NH3�����ʵ�����n(NH3)=![]() mol��1 g������ȫ�ֽ�Ϊ����������ʱ������2.72 kJ����������2 molNH3�ֽ����յ�������Q=
mol��1 g������ȫ�ֽ�Ϊ����������ʱ������2.72 kJ����������2 molNH3�ֽ����յ�������Q=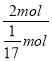 ��2.72 kJ=92.48 kJ����NH3�ֽ����N2��H2���Ȼ�ѧ����ʽΪ��2NH3(g)=N2(g)+3H2(g)����H=+92.48 kJ/mol��
��2.72 kJ=92.48 kJ����NH3�ֽ����N2��H2���Ȼ�ѧ����ʽΪ��2NH3(g)=N2(g)+3H2(g)����H=+92.48 kJ/mol��
(8)��C(s)+O2(g)=CO2(g) ��H1=��393.5 kJ/mol
��2CO(g)+O2(g)=2CO2(g) ��H2=��566 kJ/mol
��TiO2(s)+2Cl2(g)=TiCl4(s)+O2(g) ��H3=+141 kJ/mol
���ݸ�˹���ɣ�������2-��+�ۣ������ɵã�TiO2(s)+2Cl2(g)+2C(s)=TiCl4(s)+2CO(g)����H=-80 kJ/mol��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��һ�������£����淴Ӧ2A(g)![]() B(g)+3C(g)����Ӧ����ƽ��״̬���ǣ� ��
B(g)+3C(g)����Ӧ����ƽ��״̬���ǣ� ��
ѡ�� | ����Ӧ���� | �淴Ӧ���� |
A | v(A)=2mol��L-1��min-1 | v(B)=2mol��L-1��min-1 |
B | v(A)=2mol��L-1��min-1 | v(C)=2mol��L-1��min-1 |
C | v(A)=1mol��L-1��min-1 | v(B)=2mol��L-1��min-1 |
D | v(A)=1mol��L-1��min-1 | v(C)=1.5mol��L-1��min-1 |
A.AB.BC.CD.D
����Ŀ������ʵ���У���Ӧ�������Լ����۶���ȷ�����߾��������ϵ���ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ����������ϡ���Ṳ�ȡ����� | Һ�岻�ٷֲ� | ����������������������ȫˮ��ɿ��������� |
B | ������Һ�м��뱥��(NH4)2SO4��Һ | ���ɰ�ɫ���� | �����ʷ����˱��� |
C | ������ϡ�����������ˮ���������Cu(OH)2����� | ��ש��ɫ�������� | �����Ѿ���ȫˮ�� |
D | ��I-����ɫ��Һ�еμ�����������ˮ���ٵμӵ�����Һ | ������ۺ���Һ�����ɫ | �����ԣ�Cl2>I2 |
A.AB.BC.CD.D