��Ŀ����
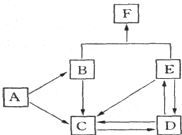
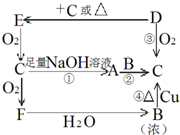
������Ԫ�γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D������E��D��������ǿ�ᣬ��������ǿ�E������������ȼ�ղ����̼�������G��G�ڴ������ܵ���������γɣ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ش��������⣺
��1����ɵ���A��Ԫ��λ�����ڱ��е�______���ڣ���______�壮
��2��B������������Һ��Ӧ�����ӷ���ʽΪ��______
��3��G�������������������·�Ӧ����������ɱ�����������ȣ��÷�Ӧ����������Ϊ______��������2mol��������ʱ��ת�Ƶ���______mol
��4����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ����ʽΪ��______��
��5��H����Һ��ϡ���ᷴӦ�ķ���ʽΪ______��
��ش��������⣺
��1����ɵ���A��Ԫ��λ�����ڱ��е�______���ڣ���______�壮
��2��B������������Һ��Ӧ�����ӷ���ʽΪ��______
��3��G�������������������·�Ӧ����������ɱ�����������ȣ��÷�Ӧ����������Ϊ______��������2mol��������ʱ��ת�Ƶ���______mol
��4����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ����ʽΪ��______��
��5��H����Һ��ϡ���ᷴӦ�ķ���ʽΪ______��
��������ǿ�ᣬ��������ǿ�ӦΪ���Ի����ΪAl��OH��3��E������������ȼ�ղ����̼�������G��G�ڴ������ܵ���������γɣ���GΪSO2��EӦΪH2S������CΪAl2S3��AΪS��BΪAl��E����������������Һ���յõ���ɫ��ҺF��ΪNa2S��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��ӦΪNa2S2����
�� 1��AΪS��ԭ������Ϊ16��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ6��λ�����ڱ��������ڵڢ�A�壬
�ʴ�Ϊ��������A��
��2��Al������������Һ��Ӧ����NaAlO2��H2����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��3��GΪSO2�����л�ԭ�ԣ����ڼ��������±�����ΪNa2SO4����Ӧ�ķ���ʽΪSO2+2NaClO3+=Na2SO4+2ClO2���ɷ���ʽ��֪��������2mol��������ʱ��ת�Ƶ���2mol��
�ʴ�Ϊ�������ƣ�Na2SO4���� 2��
��4����ҺNa2S�ڿ����г��ڷ��÷ֱ���2Na2S+O2+2H2O=4NaOH+2S��Na2S+S=Na2S2��
���4Na2S+O2+2H2O=4NaOH+2Na2S2��
�ʴ�Ϊ��4Na2S+O2+2H2O=4NaOH+2Na2S2��
��5����Һ�ɻ�ɫ��Ϊ��ɫ������dz��ɫ�����ͣ���������ζ�ģ����壬��Ӧ�����ӷ���ʽΪS22-+2H+=S��+H2S����
�ʴ�Ϊ��S22-+2H+=S��+H2S����
�� 1��AΪS��ԭ������Ϊ16��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ6��λ�����ڱ��������ڵڢ�A�壬
�ʴ�Ϊ��������A��
��2��Al������������Һ��Ӧ����NaAlO2��H2����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��3��GΪSO2�����л�ԭ�ԣ����ڼ��������±�����ΪNa2SO4����Ӧ�ķ���ʽΪSO2+2NaClO3+=Na2SO4+2ClO2���ɷ���ʽ��֪��������2mol��������ʱ��ת�Ƶ���2mol��
�ʴ�Ϊ�������ƣ�Na2SO4���� 2��
��4����ҺNa2S�ڿ����г��ڷ��÷ֱ���2Na2S+O2+2H2O=4NaOH+2S��Na2S+S=Na2S2��
���4Na2S+O2+2H2O=4NaOH+2Na2S2��
�ʴ�Ϊ��4Na2S+O2+2H2O=4NaOH+2Na2S2��
��5����Һ�ɻ�ɫ��Ϊ��ɫ������dz��ɫ�����ͣ���������ζ�ģ����壬��Ӧ�����ӷ���ʽΪS22-+2H+=S��+H2S����
�ʴ�Ϊ��S22-+2H+=S��+H2S����

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ