��Ŀ����
��嫵ĺ�����һ����ı��⣬�̺���80����Ԫ�أ��ɹ���ȡ���õ���50���֡�
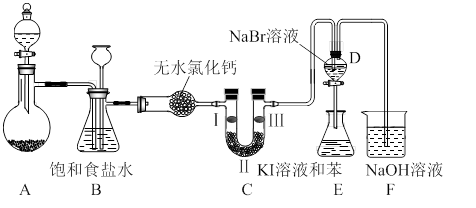
��1�����С��ȼҵ��������ʳ��ˮΪԭ����ȡCl2�����ʣ��йصĻ�ѧ����ʽΪ�� ��
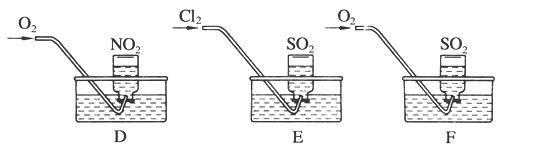
��2��ʵ�����ö���������ȡ�����Ļ�ѧ����ʽΪ�� ����������ʵ��ԭ����������װ����ѡ����ʵķ���װ������ʵ������ȡ�������� (��дװ�õ����)��
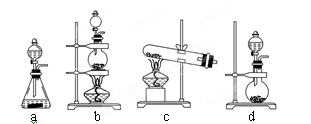
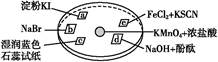
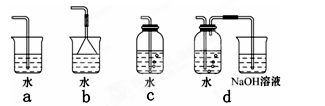
��3��ʵ������ģ��Br����Br2��ת��������ͨ����KBr��Һ�еμ�����������ˮ��ʵ�����ʵ�֡�д��Br����Br2��ת�����ӷ���ʽΪ�� ����ˮ���ȶ���Ҫ�������䣬�������������Ʊ���ˮ��װ����������� ��ѡ������ѡ��ı����ĸ����
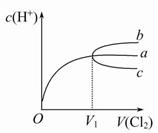
��4��ʵ�����Ʊ������ķ�Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ����������ʵ�飺ȡ100 mL����Һ�����ձ��У����ձ����������������ڵ�����ƽ�ϣ�����ͼ�����ٰ��ѳƺ�50.0 g CaCO3��ĩ�������뵽����Һ�У��ӱ߽���ʹ���ַ�Ӧ���۲�����仯���±���ʾ��

����ݴ˷������㣺
��ʵ���в�����CO2��������Ϊ ��
�ڲ���Һ����������ʵ���Ũ��Ϊ (�����ȷ��С�����һλ) ��
��1�����С��ȼҵ��������ʳ��ˮΪԭ����ȡCl2�����ʣ��йصĻ�ѧ����ʽΪ�� ��
��2��ʵ�����ö���������ȡ�����Ļ�ѧ����ʽΪ�� ����������ʵ��ԭ����������װ����ѡ����ʵķ���װ������ʵ������ȡ�������� (��дװ�õ����)��

��3��ʵ������ģ��Br����Br2��ת��������ͨ����KBr��Һ�еμ�����������ˮ��ʵ�����ʵ�֡�д��Br����Br2��ת�����ӷ���ʽΪ�� ����ˮ���ȶ���Ҫ�������䣬�������������Ʊ���ˮ��װ����������� ��ѡ������ѡ��ı����ĸ����

��4��ʵ�����Ʊ������ķ�Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ����������ʵ�飺ȡ100 mL����Һ�����ձ��У����ձ����������������ڵ�����ƽ�ϣ�����ͼ�����ٰ��ѳƺ�50.0 g CaCO3��ĩ�������뵽����Һ�У��ӱ߽���ʹ���ַ�Ӧ���۲�����仯���±���ʾ��

| CaCO3���� | δ��CaCO3ʱ | ��Լ�ķ�֮һʱ | ��һ��ʱ | ȫ������ʱ |
| ������g | 318.3 | 325.3 | 334.5 | 359.5 |
��ʵ���в�����CO2��������Ϊ ��
�ڲ���Һ����������ʵ���Ũ��Ϊ (�����ȷ��С�����һλ) ��
��1��2NaCl��2H2O 2NaOH��H2����Cl2����2�֣�
2NaOH��H2����Cl2����2�֣�
��2��MnO2��4HCl(Ũ) MnCl2��2H2O��Cl2����2�֣���b��1�֣�
MnCl2��2H2O��Cl2����2�֣���b��1�֣�
��3��2Br����Cl2��2Cl����Br2��2�֣���d��1�֣� ��4����8.8g��2�֣�����4.0mol/L��3�֣�
 2NaOH��H2����Cl2����2�֣�
2NaOH��H2����Cl2����2�֣���2��MnO2��4HCl(Ũ)
 MnCl2��2H2O��Cl2����2�֣���b��1�֣�
MnCl2��2H2O��Cl2����2�֣���b��1�֣���3��2Br����Cl2��2Cl����Br2��2�֣���d��1�֣� ��4����8.8g��2�֣�����4.0mol/L��3�֣�
�����������1�����ȼҵ������ʳ��ˮΪԭ�ϣ�ͨ����ⷨ��ȡCl2�����ʣ��йصĻ�ѧ����ʽΪ2NaCl��2H2O
 2NaOH��H2����Cl2����
2NaOH��H2����Cl2������2��ʵ�����ö���������ȡ�����Ļ�ѧ����ʽΪMnO2��4HCl(Ũ)
 MnCl2��2H2O��Cl2�������ݷ���ʽ��֪�÷�Ӧ�ǹ�����Һ������Ʊ����壬�����Ҫ����Ҫ�����Ǿƾ��ơ���Һ©������ƿ����ʵ��װ��Ӧ����װ��b������ѡb��
MnCl2��2H2O��Cl2�������ݷ���ʽ��֪�÷�Ӧ�ǹ�����Һ������Ʊ����壬�����Ҫ����Ҫ�����Ǿƾ��ơ���Һ©������ƿ����ʵ��װ��Ӧ����װ��b������ѡb����3��������������ǿ�ڵ�����ģ��ܰ��������������ɵ����壬����Br����Br2��ת�����ӷ���ʽΪ2Br����Cl2��2Cl����Br2������������ˮ�����ܽ�Ⱥ�С�����������ж�����Ҫβ������������������������Һ���գ������ȷ�Ĵ�ѡd��
��4���ٸ��������غ㶨�ɿ�֪����Ӧ�в���CO2��������318.3g��50.0g��359.5g��8.8g��
��CO2�����ʵ�����8.8g��44g/mol��0.2mol������ݷ���ʽ��֪
CaCO3��2HCl=CaCl2��H2O��CO2��
2mol 1mol
0.4mol 0.2mol
���������Ũ�ȣ�0.4mol��0.1L��4.0mol/L

��ϰ��ϵ�д�
 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
�����Ŀ