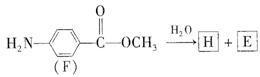��Ŀ����
��15�֣�
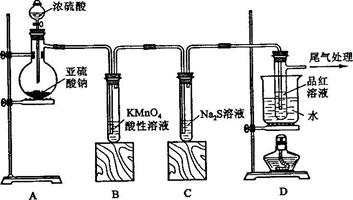
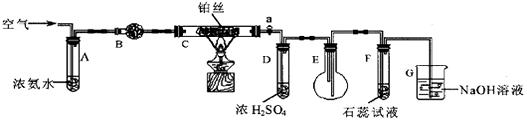
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
�뵽��F�����⣺
��1��װ��A��ʢ���������Ƶ����������� �����з����� Ӧ�Ļ�ѧ����ʽΪ ��
Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������У�װ��B��C�з���������ֱ��� �� ����Щ����ֱ�˵��SO2���е������� �� ��װ��B�з�����Ӧ�����ӷ���ʽ
Ϊ ��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ����������� ��
��4��β���ɲ��� ��Һ���ա�
��Һ���ա�
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣

�뵽��F�����⣺
��1��װ��A��ʢ���������Ƶ����������� �����з�����
 Ӧ�Ļ�ѧ����ʽΪ ��
Ӧ�Ļ�ѧ����ʽΪ ����2��ʵ������У�װ��B��C�з���������ֱ��� �� ����Щ����ֱ�˵��SO2���е������� �� ��װ��B�з�����Ӧ�����ӷ���ʽ
Ϊ ��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ����������� ��
��4��β���ɲ���
 ��Һ���ա�
��Һ���ա���1��
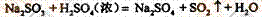
��2����Һ���Ϻ�ɫ��Ϊ��ɫ����ɫ��Һ�г��ֻ�ɫ���ǣ���ԭ�ԣ������ԣ�
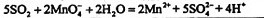
��3��Ʒ����Һ��ɫ�رշ�Һ©�������������ȼ�ƾ��Ƽ��ȣ���Һ�ָ�Ϊ��ɫ
��4��NaOH
��

��ϰ��ϵ�д�
�����Ŀ
 2NH3(g)����H=-92.2kJ��mol-1��
2NH3(g)����H=-92.2kJ��mol-1��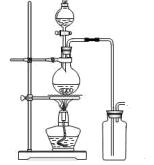
 H++HCO3- Ka1 =4��45��10-7
H++HCO3- Ka1 =4��45��10-7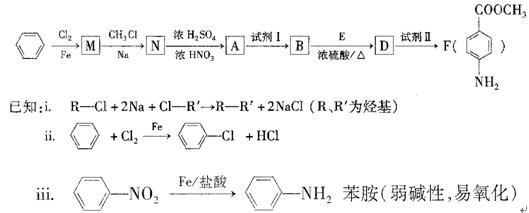
 ��������Ҫ�������D��ͬ���칹��Ľṹ��ʽһ��
��������Ҫ�������D��ͬ���칹��Ľṹ��ʽһ�� �ṹ��
�ṹ��