��Ŀ����
4����һ����NO2��N2O4�Ļ������ͨ�����Ϊ1L�ĺ����ܱ������У�������Ũ����ʱ��仯�Ĺ�ϵ��ͼ1��ʾ��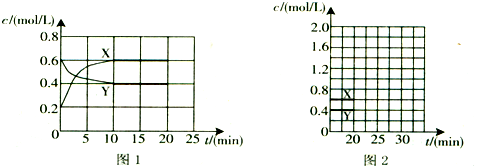
��ش�
��1������ѡ���в���˵���÷�Ӧ�Ѵﵽƽ��״̬����B����ѡ����ĸ����
A�������ڻ�������ѹǿ����ʱ��仯���ı�
B�������ڻ��������ܶȲ���ʱ��仯���ı�
C�������ڻ���������ɫ����ʱ��仯���ı�
D�������ڻ�������ƽ����Է�����������ʱ��仯���ı�
��2����Ӧ���е�10minʱ������������11.38kJ����÷�Ӧ���Ȼ�ѧ����ʽΪN2O4��g��?2NO2��g����H=+56.9kJ/mol��
��3������÷�Ӧ��ƽ�ⳣ��K=0.9��
��4����Ӧ���е�20minʱ�����������ڳ���һ����NO2��10min��ﵽ�µ�ƽ�⣬��ʱ���c��NO2��=0.9
mol/L��
�ٵ�һ��ƽ��ʱ���������NO2���������Ϊw1���ﵽ��ƽ�����������NO2���������Ϊw2����w1��w2�����������=����������
������ͼ2�л���20min������ʵ�Ũ����ʱ��仯�����ߣ������ϱ�������X���͡�Y������
���� ��ͼ1��֪��X��Y��Ũ�ȱ仯��֮��Ϊ2��1����XΪNO2��YΪN2O4��������Ӧ��N2O4��g��?2NO2��g����
��1��A���淴Ӧ���л�����������ʵ��������º����£�������ѹǿ����ѹǿ����˵������ƽ�⣻
B������������������䣬�������ݻ����䣬�ܶ�ʼ�ղ��䣻
C�������ڻ���������ɫ����ʱ��仯���ı䣬˵������������Ũ�Ȳ��䣻
D������������������䣬�淴Ӧ���л�����������ʵ�������ƽ����Է���������С��ƽ����Է�����������˵������ƽ�⣻
��2����Ӧ���е�10minʱ���μӷ�Ӧ������������Ϊ1L����0.6-0.4��mol/L=0.2mol������������11.38kJ���ʷ�Ӧ1molN2O4����������11.38kJ��5=56.9kJ��ע�����ʵľۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��3��ƽ��ʱc��NO2��=0.6mol/L��c��N2O4��=0.4mol/L������K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$���㣻
��4���ٺ��º����£��ٳ���һ����NO2����ЧΪ����ѹǿ��ƽ�������ƶ���
��20minʱ˲��c��NO2������c��N2O4�����䣬����ƽ�����淴Ӧ�����ƶ���c��NO2����С��c��N2O4������10min��ﵽ�µ�ƽ�⣬��ʱ���c��NO2��=0.9mol/L������ƽ�ⳣ������ƽ��ʱc��N2O4�����ݴ���ͼ��
��� �⣺��ͼ1��֪��X��Y��Ũ�ȱ仯��֮��Ϊ2��1����XΪNO2��YΪN2O4��������Ӧ��N2O4��g��?2NO2��g����
��1��A���淴Ӧ���л�����������ʵ��������º����£�������ѹǿ����ѹǿ����˵������ƽ�⣬��A��ȷ��
B������������������䣬�������ݻ����䣬�ܶ�Ϊ�������ܶȲ��䲻��˵������ƽ�⣬��B����
C��NO2Ϊ����ɫ���壬N2O4Ϊ��ɫ���壬�����ڻ���������ɫ����ʱ��仯���ı䣬˵������������Ũ�Ȳ��䣬��Ӧ����ƽ�⣬��C��ȷ��
D������������������䣬�淴Ӧ���л�����������ʵ�������ƽ����Է���������С��ƽ����Է�����������˵������ƽ�⣬��D��ȷ��
��ѡ��B��
��2����Ӧ���е�10minʱ���μӷ�Ӧ������������Ϊ1L����0.6-0.4��mol/L=0.2mol������������11.38kJ���ʷ�Ӧ1molN2O4����������11.38kJ��5=56.9kJ���ʸ÷�Ӧ�Ȼ�ѧ����ʽΪ��N2O4��g��?2NO2��g������H=+56.9kJ/mol��
�ʴ�Ϊ��N2O4��g��?2NO2��g����H=+56.9kJ/mol��
��3��ƽ��ʱc��NO2��=0.6mol/L��c��N2O4��=0.4mol/L����ƽ�ⳣ��K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$=$\frac{0��{6}^{2}}{0.4}$=0.9��
�ʴ�Ϊ��0.9��
��4���ٺ��º����£��ٳ���һ����NO2����ЧΪ����ѹǿ��ƽ�������ƶ�����ƽ�����������NO2�����������С����W1��W2���ʴ�Ϊ������
��20minʱ˲��c��NO2������c��N2O4�����䣬����ƽ�����淴Ӧ�����ƶ���c��NO2����С��c��N2O4������10min��ﵽ�µ�ƽ�⣬��ʱ���c��NO2��=0.9mol/L������K=$\frac{{c}^{2}��N{O}_{2}��}{c��{N}_{2}{O}_{4}��}$=0.9����ƽ��ʱc��N2O4��=$\frac{0��{9}^{2}}{0.9}$mol/L=0.9mol/L����XΪNO2��YΪN2O4��20min������ʵ�Ũ����ʱ��仯������Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���ѧƽ��״̬�жϡ�ƽ�ⳣ���ȣ���4������ͼΪ�״��㣬ע���ٴ�ƽ��ʱ�����ʵ�Ũ�ȡ������ı�˲������ʵ�Ũ�ȣ��Ѷ��еȣ�

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�| A�� | -483.6kJ•mol-1 | B�� | -241.8kJ•mol-1 | C�� | -120.6kJ•mol-1 | D�� | +241.8kJ•mol-1 |

| A�� | ����Na2CO3��Һ��CH3COOC2H5��ѡ�� | B�� | ��CCl4��ȡ��ˮ�еĵ⣬ѡ�� | ||
| C�� | ʵ����������ˮ����ȡ��ѡ�� | D�� | �����ᴿ��ѡ�ں͢� |
| A�� | �ⶨ��ҺpHʱ��pH��ֽ��������ˮ��ʪ | |
| B�� | ����ʵ���У�����ǰ������������ƿ�м������Ƭ | |
| C�� | ������ζ��ζ���ˮʵ���У��ü�����ָʾ���Լ�Сʵ����� | |
| D�� |  ����ͼ��ʾ�ķ����ų���ʽ�ζ��ܽ����е����� ����ͼ��ʾ�ķ����ų���ʽ�ζ��ܽ����е����� |
| A�� | ����FeCl3��Һ������ɫ��Ӧ | |
| B�� | �÷����к���3������̼ԭ�� | |
| C�� | �û��������Br2����ȡ����Ӧ | |
| D�� | 1mol�û�����������5molNaOH��Ӧ |
| A�� | ��Ӧ����ˮ�μӷ�Ӧ | |
| B�� | ��Ӧ����Һ����ɫ | |
| C�� | �÷�Ӧ�Ļ�ԭ��ΪK2S2O8 | |
| D�� | MnSO4��K2S2O8�Ļ�ѧ�������ֱ�Ϊ��2 |
| A�� | HCl | B�� | KOH | C�� | CaCl2 | D�� | CH4 |
| A�� | Fe2O3��Fe3O4��FeO | B�� | FeO��Fe3O4 | C�� | Fe3O4��Fe2O3 | D�� | FeO��Fe2O3 |