��Ŀ����
ijʵ��С���ù�ҵ�Ϸ������壨��Ҫ�ɷ�Cu2S��Fe2O3���������ȡ��ͭ��Fe2��SO4��3���壬��ƵIJ����������£�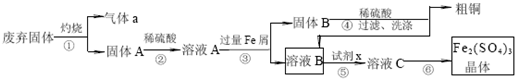
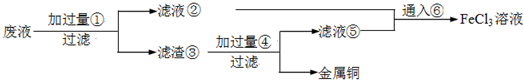
��1�����ƾ��ƺ�����̨�����ż��⣬��������������ͼг������ֱ�Ϊ �� ���ٺ͢��ж��õ�������Ϊ ��
��2���Լ�x�Ļ�ѧʽΪ ��x����ҺB��Ӧ�����ӷ���ʽΪ ��
��3��ijͬѧȡ��������ҺB�����м��������ij��ǿ���������ٵμ�KSCN��Һ��������Һ���ɫ������һ��ʱ�����Һ��ɫ����ͬѧ�²���Һ��ɫ��ԭ������Һ�е�SCN- ���������������������£�
�ָ��������Լ���1.0mol?L-1���ᡢ1.0mol?L-1 NaOH��Һ��0.1mol?L-1 Fe2��SO4��3��Һ��20%KSCN��Һ������ˮ��������ƺ���ʵ����֤��ͬѧ�IJ²��Ƿ��������Ҫ˵��ʵ�鲽������� ��
��4��ijͬѧ��ʵ���Ƶõ�Fe2��SO4��3 ��������0.1mol?L-1 ��Fe2��SO4��3 ��Һ���ڳ�����Fe2��SO4��3 ������ܽ�þ���ľ������Ϊ ��

��1�����ƾ��ƺ�����̨�����ż��⣬��������������ͼг������ֱ�Ϊ
��2���Լ�x�Ļ�ѧʽΪ
��3��ijͬѧȡ��������ҺB�����м��������ij��ǿ���������ٵμ�KSCN��Һ��������Һ���ɫ������һ��ʱ�����Һ��ɫ����ͬѧ�²���Һ��ɫ��ԭ������Һ�е�SCN- ���������������������£�
�ָ��������Լ���1.0mol?L-1���ᡢ1.0mol?L-1 NaOH��Һ��0.1mol?L-1 Fe2��SO4��3��Һ��20%KSCN��Һ������ˮ��������ƺ���ʵ����֤��ͬѧ�IJ²��Ƿ��������Ҫ˵��ʵ�鲽�������
��4��ijͬѧ��ʵ���Ƶõ�Fe2��SO4��3 ��������0.1mol?L-1 ��Fe2��SO4��3 ��Һ���ڳ�����Fe2��SO4��3 ������ܽ�þ���ľ������Ϊ
��������1���ٹ��������õ����������ͼг���������ǯ�������ղ����͢���ҺŨ�����������������ж��õ��IJ�������
��2 �������Լ�x�����������ӣ�ʹ���Լ����������µ����ʣ�����ѡ��˫��ˮ������������Ӧ����ʽ��H2O2 2Fe2++H2O2 +2H+=2Fe3++2H2O��
��3��ͨ�����µμ����軯����Һ��֤����ͬѧ�ļ����Ƿ������
��4��������������Һ��Ҫ��ֹ�����ӵ�ˮ�⣬��Ҫ��������ϡ���ᣮ
��2 �������Լ�x�����������ӣ�ʹ���Լ����������µ����ʣ�����ѡ��˫��ˮ������������Ӧ����ʽ��H2O2 2Fe2++H2O2 +2H+=2Fe3++2H2O��
��3��ͨ�����µμ����軯����Һ��֤����ͬѧ�ļ����Ƿ������
��4��������������Һ��Ҫ��ֹ�����ӵ�ˮ�⣬��Ҫ��������ϡ���ᣮ
����⣺��1�������չ���Ӧ�÷��������У�����������ǯ�г֣������ղ����͢���ҺŨ���������������嶼�õ���������
�ʴ�Ϊ������������ǯ�� ��������
��2�����ڼ����Լ�xĿ���������������ӣ�ʹ���Լ����������µ����ʣ�����ѡ��˫��ˮ������������Ӧ����ʽ��2Fe2++H2O2 +2H+=2Fe3++2H2O��
�ʴ�Ϊ��H2O2��2Fe2++H2O2 +2H+=2Fe3++2H2O��
��3�����¼������軯����Һ��������Һ��죬˵����ͬѧ�²������������������Ϊ��ȡ������ɫ�����Һ���Թ��У���μ��������20%KSCN��Һ������Һ���ɫ��˵����ͬѧ�IJ²��Ǻ����ģ��粻���ɫ��˵���²ⲻ������
�ʴ�Ϊ��ȡ������ɫ�����Һ���Թ��У���μ��������20%KSCN��Һ������Һ���ɫ��˵����ͬѧ�IJ²��Ǻ����ģ��粻���ɫ��˵���²ⲻ��������μӹ�����0.1 mol?L-1 Fe2��SO4��3��Һ������Һ�����ɫ��˵�������������ɫ��˵���²ⲻ��������
��4����������������Һʱ����Ҫ����ϡ�����ֹ�����ӵ�ˮ�⣬�������Ʒ���Ϊ���������ľ��������ձ��У�����������ϡ���������ˮ���ò��������裬
�ʴ�Ϊ���������ľ��������ձ��У�����������ϡ���������ˮ���ò��������裮
�ʴ�Ϊ������������ǯ�� ��������
��2�����ڼ����Լ�xĿ���������������ӣ�ʹ���Լ����������µ����ʣ�����ѡ��˫��ˮ������������Ӧ����ʽ��2Fe2++H2O2 +2H+=2Fe3++2H2O��
�ʴ�Ϊ��H2O2��2Fe2++H2O2 +2H+=2Fe3++2H2O��
��3�����¼������軯����Һ��������Һ��죬˵����ͬѧ�²������������������Ϊ��ȡ������ɫ�����Һ���Թ��У���μ��������20%KSCN��Һ������Һ���ɫ��˵����ͬѧ�IJ²��Ǻ����ģ��粻���ɫ��˵���²ⲻ������
�ʴ�Ϊ��ȡ������ɫ�����Һ���Թ��У���μ��������20%KSCN��Һ������Һ���ɫ��˵����ͬѧ�IJ²��Ǻ����ģ��粻���ɫ��˵���²ⲻ��������μӹ�����0.1 mol?L-1 Fe2��SO4��3��Һ������Һ�����ɫ��˵�������������ɫ��˵���²ⲻ��������
��4����������������Һʱ����Ҫ����ϡ�����ֹ�����ӵ�ˮ�⣬�������Ʒ���Ϊ���������ľ��������ձ��У�����������ϡ���������ˮ���ò��������裬
�ʴ�Ϊ���������ľ��������ձ��У�����������ϡ���������ˮ���ò��������裮
��������������Ȼ����������ȡ��������ʵ��������������ơ��Լ�ѡ����������Һ���ơ����鷽�����Լ������ӷ���ʽ��д��֪ʶ����Ŀ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ��������������������Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
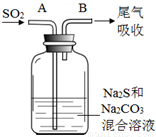
ij����С��Ӻ��н϶�Ag+��Fe3+��Al3+�Ĺ�ҵ��ˮ�У�����ͼ��ʾ�����������ȡ�������ʣ�
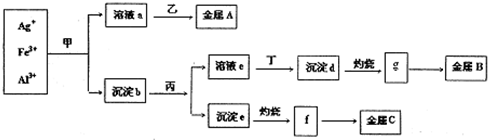
��֪��ʵ���Ҽס��ҡ���������������ֻ�ܴӰ�ˮ��̼��李��������ơ�ϡ���ᡢ������������Һ��ѡ��Ҳ��������������Һ���Ʊ����Ը�ʵ�������ȷ���ǣ�������

��֪��ʵ���Ҽס��ҡ���������������ֻ�ܴӰ�ˮ��̼��李��������ơ�ϡ���ᡢ������������Һ��ѡ��Ҳ��������������Һ���Ʊ����Ը�ʵ�������ȷ���ǣ�������
| A������A��B��C�ֱ�ΪAg��Fe��Al | B��g��f��Ϊ�������ҵ�Ͼ����õ��g��f��ö�Ӧ�������� | C������bΪ������Ҫ�ɷ�Ϊ����d�ͳ���e | D�������������ƣ���Ϊϡ���� |
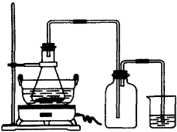 �̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ���
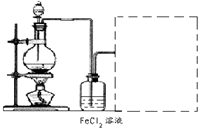
�̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ�����ش��������⣺
��1��ͼ��ij�о���ѧϰС�������Ʊ��̷���װ�ã����м���ƿ��������
��2����ͼ��ƿ�еķ�Ӧ������м��28%�����ᣬ��Ӧǰ����98%����������500g 28%�����ᣬҪ�������98%��������������Ҫ֪����������
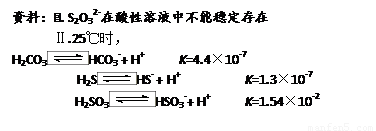
��3�����Ʊ����������Ĺ����У������Ͽ�����������������������ڷ�ֹFe2+���������������ʵ�飺ʵ��һ�������������ʵ��������������������ಽ��ͬ���Ƶ��������������Բ�Ʒ�������м�⣮��������£�
| ��� | ��Ӧ������ | ��Ʒ���� |
| 1 | n��H2SO4����n��Fe����1��1 | ���ڢ���֮�� |
| 2 | n��H2SO4����n��Fe����1��1 | ���ڢ� |
��4����С���ڼ����о����̷��Ʊ�Fe��OH��2�Ĺ����У����ֳ��ְ�ɫ�ij�������ת��Ϊ����ɫ������Ϊ���ɫ����ͬѧ�����ϸû���ɫ����������Fe3��OH��8���������������ʽд���ó���