��Ŀ����
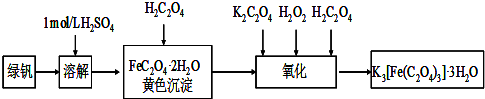
 �̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ���
�̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ�����ش��������⣺
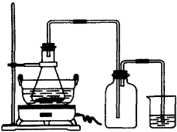
��1��ͼ��ij�о���ѧϰС�������Ʊ��̷���װ�ã����м���ƿ��������
��2����ͼ��ƿ�еķ�Ӧ������м��28%�����ᣬ��Ӧǰ����98%����������500g 28%�����ᣬҪ�������98%��������������Ҫ֪����������
��3�����Ʊ����������Ĺ����У������Ͽ�����������������������ڷ�ֹFe2+���������������ʵ�飺ʵ��һ�������������ʵ��������������������ಽ��ͬ���Ƶ��������������Բ�Ʒ�������м�⣮��������£�
| ��� | ��Ӧ������ | ��Ʒ���� |
| 1 | n��H2SO4����n��Fe����1��1 | ���ڢ���֮�� |
| 2 | n��H2SO4����n��Fe����1��1 | ���ڢ� |
��4����С���ڼ����о����̷��Ʊ�Fe��OH��2�Ĺ����У����ֳ��ְ�ɫ�ij�������ת��Ϊ����ɫ������Ϊ���ɫ����ͬѧ�����ϸû���ɫ����������Fe3��OH��8���������������ʽд���ó���
��������1������װ��ͼ����������ƿ����ȫƿ�����÷����жϣ�����������ԭ��Ӧ��Ԫ�ػ��ϼ۱仯�͵����غ����������ƽд����ѧ����ʽ��
��2������m=��V���жϣ�
��3�����ݱ����в�Ʒ�ȼ��ͷ�Ӧ�������жϣ�
��4��������Ԫ�صĻ��ϼۣ�������Fe3��OH��8����Ԫ�ػ��ϼ��ܺ�ӦΪ+8�����ƶϸ����ʵķ����к�+2��Fe2+һ����+3��Fe3+��������������Ԫ������Ԫ�ؽ�ϳ����������д�仯ѧʽ�����ݳ����ɡ��ס��䡰�̡�������������Fe��OH��2���ֱ�����������������Fe��OH��3��Ե�ʷ�������˿ɲ�ȡ��Fe2+��Fe3+�Ļ��Һ�м���NaOH��Һ�����������ֱ�Ӱ��������ʻ�ϣ��۲���ɫ�ķ�������֤�����ɡ��ס��䡰�̡���ԭ��
��2������m=��V���жϣ�
��3�����ݱ����в�Ʒ�ȼ��ͷ�Ӧ�������жϣ�
��4��������Ԫ�صĻ��ϼۣ�������Fe3��OH��8����Ԫ�ػ��ϼ��ܺ�ӦΪ+8�����ƶϸ����ʵķ����к�+2��Fe2+һ����+3��Fe3+��������������Ԫ������Ԫ�ؽ�ϳ����������д�仯ѧʽ�����ݳ����ɡ��ס��䡰�̡�������������Fe��OH��2���ֱ�����������������Fe��OH��3��Ե�ʷ�������˿ɲ�ȡ��Fe2+��Fe3+�Ļ��Һ�м���NaOH��Һ�����������ֱ�Ӱ��������ʻ�ϣ��۲���ɫ�ķ�������֤�����ɡ��ס��䡰�̡���ԭ��
����⣺��1��װ��ͼ�з�����֪������ƿ��ȫƿ�����ã���ֹ�ձ��е�Һ�嵹������ƿ����Ⱦ���ɵIJ��������ԭ��Ӧ��CuSO4��ͭԪ�ػ��ϼ۴�+2�۱仯Ϊ0�ۣ�ת�Ƶ���Ϊ2��PH3����Ԫ�ػ��ϼ�-3�۱仯Ϊ+5�ۣ�ת�Ƶ���Ϊ8�����ݵ����غ��ԭ���غ���ƽ��д�õ���ѧ����ʽΪ��4CuSO4+PH3+4H2O=H3PO4+4H2SO4+4Cu
�ʴ�Ϊ����ȫƿ���ã���ֹ�ձ��е�ϴҺ��������ƿ����Ⱦ���4��1��4H2O��1��4��4��
��2������m=��V��֪��Ҫ������Һ���������Ҫ֪��98%������ܶȣ��ʴ�Ϊ��98%������ܶȣ�
��3�����������������ȷ���˲�Ʒ�ļ��𣬴�Fe3+��������������0.005%����Ʒ������Ϊ��Fe3+��������������0.01%����Ʒ������Ϊ�����Կ������������ĺ���Խ�ͼ���Խ�ߣ��ӱ��п��Կ�����ֻҪ��������������Ʒ����ߣ��ʴ�Ϊ������
��4����������Ϣ��֪������������Ϊ��ɫ����������Ϊ��ɫ�����ߵĻ������ֳ���ɫ�����ʵ���г��ֻ���ɫ����ɫ������Ӧ�����ɵ�Fe��OH��2���ֱ�����������������Fe��OH��3��Ե�ʣ��������ἰ����ɫFe3��OH��8��������ΪFe��OH��2��Fe��OH��3�Ľ�����һ�����ʣ����м���+2����Ҳ��+3��������˸�дΪ���������ʽʱ��8����ԭ��Ӧ��ϳ�4H2O������Ӧд�ɣ�FeO?Fe2O3?4H2O�����ڳ��ֵij����ɰױ�������Ϊ�γ���Fe��OH��2��Fe��OH��3�Ļ�ϳ�������˿�������Fe2+��Fe3+�Ļ��Һ��ֱ�Ӽ���NaOH��Һ����Ӧ����Fe��OH��2��Fe��OH��3�Ļ�ϳ�����۲��ʱ���������ɫ����ֱ��ȡFe��OH��2��Fe��OH��3���л�ϣ��۲��Ϻ���ɫ�ķ������Խ��ͳ����ɡ��ס��䡰�̡�������
�ʴ�Ϊ��FeO?Fe2O3?4H2O����Fe2+��Fe3+�Ļ��Һ�м���NaOH��Һ�۲����ɵij�������ɫ�Ƿ�Ϊ����ɫ�����߿��Խ�Fe��OH��3��Fe��OH��2��Ϻ�۲�����ɫ
�ʴ�Ϊ����ȫƿ���ã���ֹ�ձ��е�ϴҺ��������ƿ����Ⱦ���4��1��4H2O��1��4��4��
��2������m=��V��֪��Ҫ������Һ���������Ҫ֪��98%������ܶȣ��ʴ�Ϊ��98%������ܶȣ�
��3�����������������ȷ���˲�Ʒ�ļ��𣬴�Fe3+��������������0.005%����Ʒ������Ϊ��Fe3+��������������0.01%����Ʒ������Ϊ�����Կ������������ĺ���Խ�ͼ���Խ�ߣ��ӱ��п��Կ�����ֻҪ��������������Ʒ����ߣ��ʴ�Ϊ������
��4����������Ϣ��֪������������Ϊ��ɫ����������Ϊ��ɫ�����ߵĻ������ֳ���ɫ�����ʵ���г��ֻ���ɫ����ɫ������Ӧ�����ɵ�Fe��OH��2���ֱ�����������������Fe��OH��3��Ե�ʣ��������ἰ����ɫFe3��OH��8��������ΪFe��OH��2��Fe��OH��3�Ľ�����һ�����ʣ����м���+2����Ҳ��+3��������˸�дΪ���������ʽʱ��8����ԭ��Ӧ��ϳ�4H2O������Ӧд�ɣ�FeO?Fe2O3?4H2O�����ڳ��ֵij����ɰױ�������Ϊ�γ���Fe��OH��2��Fe��OH��3�Ļ�ϳ�������˿�������Fe2+��Fe3+�Ļ��Һ��ֱ�Ӽ���NaOH��Һ����Ӧ����Fe��OH��2��Fe��OH��3�Ļ�ϳ�����۲��ʱ���������ɫ����ֱ��ȡFe��OH��2��Fe��OH��3���л�ϣ��۲��Ϻ���ɫ�ķ������Խ��ͳ����ɡ��ס��䡰�̡�������
�ʴ�Ϊ��FeO?Fe2O3?4H2O����Fe2+��Fe3+�Ļ��Һ�м���NaOH��Һ�۲����ɵij�������ɫ�Ƿ�Ϊ����ɫ�����߿��Խ�Fe��OH��3��Fe��OH��2��Ϻ�۲�����ɫ
���������⿼���������仯�������ʵķ�������Ӧ������ж�Ӧ�ã�����ʵ�����ṩ���ϵķ�������ȷ����ʵ���Ŀ�ļ����������ԭ���ڽ�����֪ʶ��̽����������������Ҫ�ģ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ