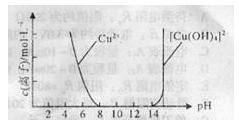
��Ŀ����
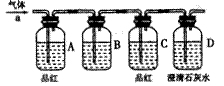
(12��) ijʵ��С������ͼװ�ý����Ҵ���������ʵ�顣
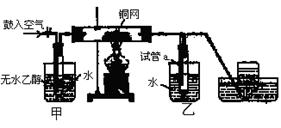
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ�� ��Ӧ�����Ȼ����ȣ���
(2)��������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ������� ,
����ƿ���ռ������������Ҫ�ɷ��� ��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� (��д��ĸ)��Ȼ����ͨ�� (��ʵ���������)���ɳ�ȥ��
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼

(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ�� ��Ӧ�����Ȼ����ȣ���
(2)��������ˮԡ���ò���ͬ���������� ��
�ҵ������� ��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ������� ,
����ƿ���ռ������������Ҫ�ɷ��� ��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� (��д��ĸ)��Ȼ����ͨ�� (��ʵ���������)���ɳ�ȥ��
a���Ȼ�����Һ b���� c��̼��������Һ d�����Ȼ�̼
��12�֣�(1)2Cu+O2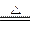 2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO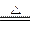 CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
2CH3CH2OH + O2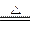 2CH3CHO + 2H2O ����
2CH3CHO + 2H2O ����
(2)������ȴ (3)��ȩ �Ҵ� ˮ ���� (4) ���� c ����
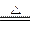 2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO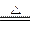 CH3CHO+Cu+H2O
CH3CHO+Cu+H2O 2CH3CH2OH + O2
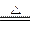 2CH3CHO + 2H2O ����
2CH3CHO + 2H2O ���� (2)������ȴ (3)��ȩ �Ҵ� ˮ ���� (4) ���� c ����
��

��ϰ��ϵ�д�
�����Ŀ



 ��
��
 2NH3(g)+CO2(g)
2NH3(g)+CO2(g)
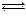
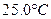 ʱ��0-6min ���������ˮ�ⷴӦ��ƽ������ ______��
ʱ��0-6min ���������ˮ�ⷴӦ��ƽ������ ______��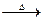 CH3COOH+Cu2O��+2H2O����������ɣ�����������̽����
CH3COOH+Cu2O��+2H2O����������ɣ�����������̽����