��Ŀ����
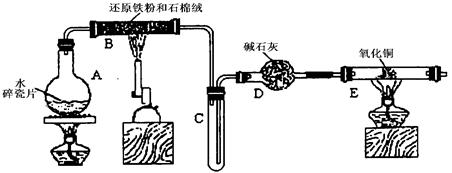
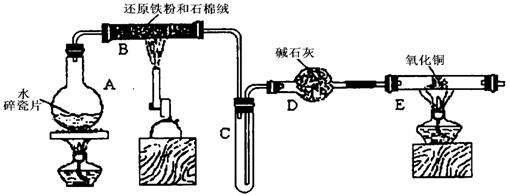 ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��
(1) ��Ӳ�ʲ�����B���ȵ�����������_________
(2)B�з�����Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
(3)Ϊ�˰�ȫ������E��ǰ������еIJ�����________________________________________��
(4)��֪�з�Ӧ��Cu20+2H+![]() Cu+Cu2++H20������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu20�����õ��Լ���________(�����)
Cu+Cu2++H20������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu20�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(5)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɻ�ͭ�����ԭ������������Ӧǰʢ��ҩƷ��E��F�������ֱ���bg��cg����Ӧ��ֱ���dg��eg��
��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(0)= ________:_______������Ӧ��E�г�Cu��������ֻ�ԭ����Cu20����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
�����յ�E�ܵ�����Ϊag�����Ⱥ�CuO��ȫ��ԭΪCu����ͭ�����ԭ�������ɱ�ʾΪ______________________________��
��6��ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��____________����Ϊ�������������Ե�������
��1���ƾ����
��2��3Fe��4H2O������ ![]() Fe3O4��4H2
Fe3O4��4H2
��3����E�ij����ռ������鴿�������װ���������Ĵ��ȣ�
��4��b��c
��5��(c-e)-(b-d):(b-d)/16����Ӱ�죻16��d-a��/��b-d��
��6��ͭ6.4g��������ͭ7.2g

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�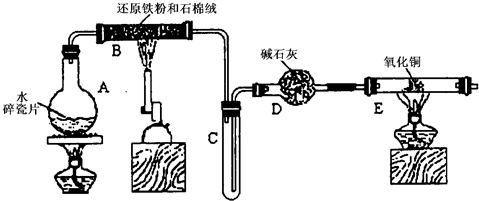
 Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)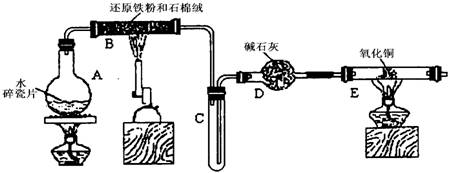
 Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)