��Ŀ����
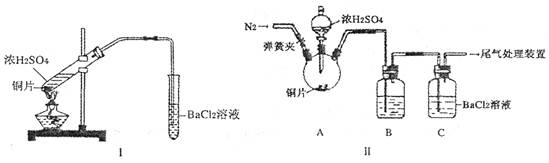
(12��)ijѧϰС��������ͼ��ʾװ�ý��С�����ˮ����Ӧ��������ʵ��(��ȥ�˼г�����)��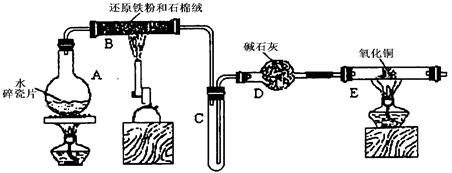
(1)Ϊ�˰�ȫ������E��ǰ������еIJ�����____________________________��
(2) B�з�����Ӧ�Ļ�ѧ����ʽ�� _______________��
(3)��֪�з�Ӧ��Cu2O+2H+ Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
a��ϡ���� b��ϡ���� c������ d��Ũ����
(4)��E�����ٽӶ���ʢ��ʯ�ҵĸ����(���γ�ΪF��G)�����ø�װ�òⶨˮ����ɡ�����Ӧ��ʢ��ҩƷ��E������������e g��F������������f g��ˮ��Ԫ�ص����ʵ���֮�ȿɱ�ʾΪ�����û���n(H)��n(O)= ________��_______������Ӧ��E�г�Cu�������һ�ֻ�ԭ����Cu2O����ñ�ֵ��___________ (ѡ�ƫ��ƫС������Ӱ�족)��
(5)ijѧ��Ϊ�˼��鷴Ӧ�����ijɷ֣���Ӧǰ��E���м���������ͭ����16g����Ӧ����������������Ϊ13.6g����13.6g�ijɷ�Ϊ��___________����Ϊ�������������Ե�������
(12��)
����E�ij����ռ������鴿�������װ���������Ĵ��ȣ���2�֣�
��3Fe��4H2O������ Fe3O4��4H2��2�֣�
Fe3O4��4H2��2�֣�
��b��c��1�֣�
��f-e��e/16��2�֣�����Ӱ�죨1�֣���
��5��ͭ6.4g��������ͭ7.2g��4�֣�
����

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� ���µ����⣬̽��������Э����С���ͬѧ��������о���
���µ����⣬̽��������Э����С���ͬѧ��������о���
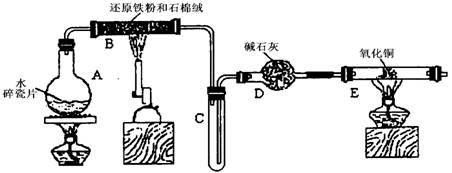
 �ܱ�NH3��ԭ��
�ܱ�NH3��ԭ�� ��ɫ������Cu��Ҳ��ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ����
��ɫ������Cu��Ҳ��ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ���� ��Cu��A�Ļ����������һ����ʵ�����NH3��CuO��Ӧ�����ɵĺ�ɫ�������Ƿ���A�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Cu��A�Ļ����������һ����ʵ�����NH3��CuO��Ӧ�����ɵĺ�ɫ�������Ƿ���A�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ� Y��SO2�ĺ���������������ַ�����
Y��SO2�ĺ���������������ַ�����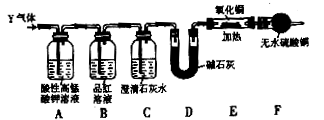
 ��������������
�������������� ��
��
 Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)
Cu+Cu2++H2O������������鷴Ӧ��E�еĺ�ɫ�����г�Cu���Ƿ�Cu2O�����õ��Լ���________(�����)