��Ŀ����
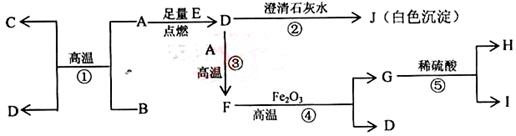
��14�֣�A��B��C��D��G���Ǻ���ͬһ�ֶ�����Ԫ�صĻ����E�������H�ǵ��ʡ�������ͼת����ϵ����Ӧ���������ֲ�������ȥ����գ�&s.5*u.c.om
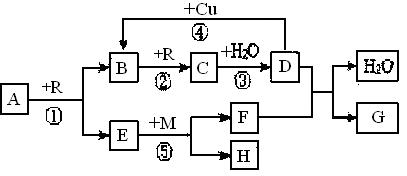
��1����A��B��C��D��G ����XԪ�أ���A��һ��������ֻ����10�����ӣ�G�Ǹ��Ϸ��ϣ���Ӧ�١��ڡ����ǹ�ҵ���� �Ļ���ԭ����M�Ļ�ѧʽΪ �����з�Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ܵ����ӷ���ʽΪ ��
��2����A��B��C��D��G����YԪ�أ�YԪ�ش���X���������ڡ������壬G������ˮ������Ӧ�١��ڡ��۾��ǹ�ҵ���� �Ļ���ԭ����M�γɵľ�������Ϊ �����з�Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ݵĻ�ѧ����ʽΪ ��

��1����A��B��C��D��G ����XԪ�أ���A��һ��������ֻ����10�����ӣ�G�Ǹ��Ϸ��ϣ���Ӧ�١��ڡ����ǹ�ҵ���� �Ļ���ԭ����M�Ļ�ѧʽΪ �����з�Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ܵ����ӷ���ʽΪ ��
��2����A��B��C��D��G����YԪ�أ�YԪ�ش���X���������ڡ������壬G������ˮ������Ӧ�١��ڡ��۾��ǹ�ҵ���� �Ļ���ԭ����M�γɵľ�������Ϊ �����з�Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ݵĻ�ѧ����ʽΪ ��
��1������(1��)��K��K2O2��KO2��(2��)
4NH3+5O2
3Cu+8H++2NO3- 3Cu2++2NO��+4H2O(2��)
��2������(1��)���������� (2��) 4FeS2+11O2 2Fe2O3+8SO2 (2��)
Fe2O3 + 2Al Al2O3+ 2Fe (2��&s.5*u.c.om)
Al2O3+ 2Fe (2��&s.5*u.c.om)
4NH3+5O2

3Cu+8H++2NO3-
��2������(1��)���������� (2��) 4FeS2+11O2
Fe2O3 + 2Al
 Al2O3+ 2Fe (2��&s.5*u.c.om)
Al2O3+ 2Fe (2��&s.5*u.c.om)��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
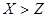
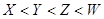
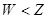
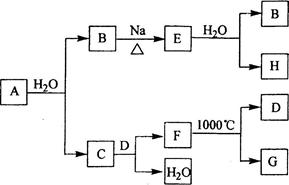

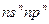 ��CԪ�صĵ�һ��������ͬ��Ԫ��������Ҹ���ͬ�����������ڵ�Ԫ�أ���
��CԪ�صĵ�һ��������ͬ��Ԫ��������Ҹ���ͬ�����������ڵ�Ԫ�أ���