��Ŀ����
����Ŀ����1��һ��������1 L���ܱ������У���ӦmA(g)+nB(g) ![]() pC(g)+qD(g)�ﵽƽ�⡣����ʼʱAΪ1 mol����Ӧ2min�ﵽƽ�⣬Aʣ��0.4 mol������0~2min��A��ƽ����Ӧ����Ϊ______mol/(Lmin)��
pC(g)+qD(g)�ﵽƽ�⡣����ʼʱAΪ1 mol����Ӧ2min�ﵽƽ�⣬Aʣ��0.4 mol������0~2min��A��ƽ����Ӧ����Ϊ______mol/(Lmin)��
��2���������������������£����������������ƽ�����淴Ӧ�����ƶ���m+n______p+q��ѡ�>������<����=������v��______��ѡ���������С�����䡱����
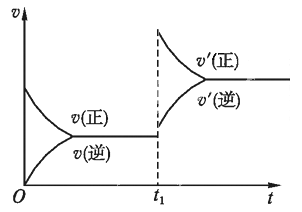
��3������Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������t1ʱ�����ʷ����仯��ԭ�������________����ѡ���ţ���ͬ��
a.����A��Ũ�� b. ��С�������
c. ������� d.�����¶�
��4����nV��(A)=mV��(B)����÷�Ӧ________��
a.������Ӧ������� b. ���淴Ӧ������� c.����ƽ��״̬ d.���ж�
���𰸡� 0.3mol/(L�� min) m+n>p+q ��С bd c
�����������⿼�黯ѧ��Ӧ���ʵļ��㡢Ӱ�컯ѧƽ���ƶ������ء���ѧƽ��״̬���жϣ���1�����ݻ�ѧ��Ӧ���ʵ���ѧ����ʽ��v(A)=(1��0.4)/2mol/(L��min)=0.3 mol/(L��min)����2���������������ѹǿ��С��ƽ�����淴Ӧ�����ƶ���˵����Ӧǰ����ϵ��֮�ʹ��ڷ�Ӧ������ϵ��֮�ͣ���m��n>p��q����Сѹǿ����ѧ��Ӧ���ʽ��ͣ���v���С����3��t1ʱ�����淴Ӧ���ʶ����ı�����������������¶Ȼ�����ѹǿ����bd��ȷ����4���������ʵķ�����һ��������Ӧ������У�һ�����淴Ӧ������У������ǵ�����֮�ȵ���m��n�����ڻ�ѧ������֮�ȣ���˵����Ӧ�ﵽƽ�⣬��c��ȷ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�