��Ŀ����
����Ŀ��298Kʱ������(HCOOH)�ͼ����ƵĻ����Һ��HCOOH��HCOO��Ũ�ȴ��ڹ�ϵʽc(HCOO)+c(HCOOH)=0.100mol��L1������̼Ԫ�ص����ӵ�Ũ����pH�Ĺ�ϵ��ͼ��ʾ��

����˵����ȷ����( )
A.0.1mol��L1HCOONa��Һ����c(HCOO)+c(HCOOH)+c(OH)=c(H+)+0.1
B.298Kʱ��HCOOH�ĵ��볣��Ka=1.0��103.75
C.298Kʱ��������ˮϡ��P����Һ����Һ��n(H+)��n(OH)���ֲ���
D.0.1mol��L1HCOONa��Һ��0.1mol��L1HCOOH��Һ�������Ϻ���Һ��pH=3.75(��Ϻ���Һ����仯���Բ���)
���𰸡�B
��������
A��0.1mol��L1HCOONa��Һ�����ˮ����Һ�Լ���c(OH)��c(H+)�����������غ��֪c(HCOO)+c(HCOOH)=c(Na+)=0.1mol��L1�����c(HCOO)+c(HCOOH)+c(OH)��c(H+)+0.1mol��L1��A����
B������ͼ���֪P��ʱ298Kʱc(HCOO)��c(HCOOH)��c(H+)��10��3.75mol��L1����HCOOH�ĵ��볣��Ka=![]() =c(H+)=1.0��103.75��B��ȷ��
=c(H+)=1.0��103.75��B��ȷ��
C��298Kʱ��������ˮϡ��P����Һ����Һ�������ӻ������������ʵ������ӣ���Һ��n(H+)��n(OH)����C����
D��0.1molL-1HCOONa��Һ��0.1molL-1HCOOH��Һ�������Ϻ���Ȼ�����Һ��c(HCOO-)+c(HCOOH)=0.100molL-1��������HCOOH�ĵ���̶ȴ���HCOONa��ˮ��̶ȣ������Һ��c(HCOO-)��c(HCOOH)������Һ��pH��3.75��D����
��ѡB��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�����Ŀ��ZnSO47H2O�����ڲⶨ������¯���ĺ�������ij�����½����к�п����(Cd)��ͭ�����Ƚ������Ը��½���Ϊԭ���Ʊ�ZnSO47H2O������Cu��Cd�Ĺ���������ͼ��ʾ��
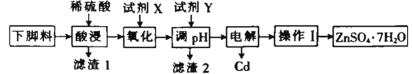
��֪�����������↑ʼ����ȫ������pH�����ʾ(��ʼʱ��������Ũ�Ȱ�0.1molL-1����)��
�������� | Fe(OH)3 | Cd(OH)2 | Zn(OH)2 |
��ʼ������ pH | 1.9 | 7.4 | 6.2 |
��ȫ������ pH | 3.2 | 9.5 | 8.2 |
��ش��������⣺
(1)����1�к���_______(�ѧʽ)��
(2)�Լ�X��˫��ˮʱ��д�����������з�����Ӧ�����ӷ���ʽ_______��
(3)pH�ĵ��ط�Χ��_______���Լ�Y��ѡ��_______(����ĸ)
A. NaOH B. ZnO C. ZnCO3
(4)���ʱ�Թ�(Hg)���缫���Խ�����п��ȫ���룬�ӵ�����_______(����������������)�������������ĵ缫��ӦʽΪ_______��
(5)����I�����IJ�����_______�����˵ȣ����˺�õ�����Һ���Լ���_______ (�������������������)����ѭ�����á�