��Ŀ����
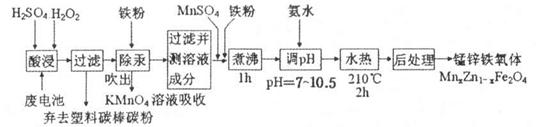
K2SO4���������ʼطʣ�Mn3O4������������������ϵ���Ҫԭ�ϡ������Ṥҵ��β�������Ʊ�K2SO4��Mn3O4�Ĺ����������£�
��1�������ε��ܽ�ȼ�ͼ����ӦIII�У���(NH4)2SO4��Һ�м���KCl��Һ��ַ�Ӧ��������Ũ���� ��ϴ�ӡ�����Ȳ�������K2SO4��Ʒ��
��2������K2SO4��Ʒ�Ƿ����Ȼ������ʵ�ʵ������ǣ� ��
��3����ӦIV�Ļ�ѧ����ʽΪ ��
��4��Mn3O4��Ũ�������ʱ������Ӧ�����ӷ���ʽΪ ��
��5����ͼ������MnSO4?H2Oʱ�¶���ʣ����������仯���ߡ�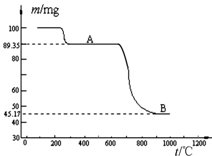
�ٸ�������B������ʾ���ʵĻ�ѧʽΪ ��
�����չ����й����̺������¶ȵ����߶��������¶ȳ���1000��ʱ������ȴ��ò�������̺���������С���Է����������̺�����С��ԭ�� ��
��1�����ȹ���
��2��ȡ������Ʒ���Թ��������Һ���μӹ���Ba(NO3)2��Һ��ȡ�ϲ���Һ�μ�AgNO3��Һ
��3��MnO2��SO2��MnSO4
��4��Mn3O4��8H����2Cl�� 3Mn2����Cl2����4H2O
3Mn2����Cl2����4H2O
��5����Mn3O4�ڲ���Mn3O4�ֱ�����ΪMn2O3��MnO2��������̺�����С
���������������1��������������ͬ�¶���������ܽ����С�����Է�ӦIII������Ũ������������������ȹ��˵�����ؾ��壬��ϴ�ӡ����T�ɡ���2������Cl-һ������������Һ��ϡ���ᣬ��SO42-Ҳ����Ag+�γ����������������Լ���Cl-ǰҪ�ȼӹ������ᱵ��Һ��SO42-���������˺�����Һ�м���������Һ���Ƿ������ɫ��������3����������������������������̡���4��Mn������̬��+2��+4��+6��+7�����������̿ɸ�дΪMnO2��2MnO��MnO2��Ũ��������MnCl2��Cl2��H2O��MnO����������MnCl2��H2O����5��������ͼ��֪�����̾�����յõ����������̣�����ͼ�����ݣ���Mn�غ�ȷ��BΪ���������̡��¶ȳ���1000��ʱ������ȴ��ò�������̺���������Сԭ����������������̱�����Ϊ�������̻����������̣�ʹ�̺�����С��
���㣺 ��ѧʵ�� ���ӵļ��� ��ѧ�����ӣ�����ʽ����д ͼ��ķ���

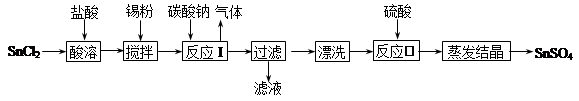
��ʯ�����ȼҵ�е�һ�ַ���������������±���ʾ��
�õ�ʯ����������ˮCaCl2��ij��������������¹������̣�
��֪�Ȼ��ƾ���Ļ�ѧʽ�ǣ�CaCl2��6H2O��H2S��һ���������壬�Ҿ��л�ԭ�ԡ�
��1����Ӧ���м������Ӧѡ��___________________��
��2����ɫ����Ӧ���������X��_______________���豸A��������______________���豸B������Ϊ________________���豸C��������____________________��
��3��Ϊ�����㻷��Ҫ���轫����H2Sͨ�����ճأ��������������ʺ���Ϊ���ռ�����_____________����Ӧ�Ļ�ѧ����ʽΪ_________________��
| A��ˮ | B��Ũ���� | C��ʯ���� | D������ |
��5���ȼҵ�缫����ʽ_____________________��
�ϳɰ���ҵ�ϲ���ѭ��������ԭ����Ҫ��( )
| A���ӿ췴Ӧ���� |
| B�������NH3��ƽ��Ũ�� |
| C������NH3�ķе� |
| D�����N2��H2�������� |
��ҵ���������ʵ�ѭ�������ж���ģʽ�����磺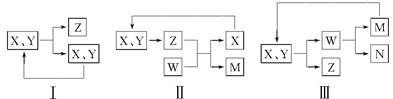
���б�����ȷ���� (����)��
| A��ͼ������ںϳɰ���N2��H2��ѭ�� |
| B��ͼ������ڰ���ƴ�����CO2��ѭ�� |
| C��ͼ������ڵ�ⱥ��ʳ��ˮ��NaCl��ѭ�� |
| D��ͼ������ڰ���������������NO��ѭ�� |
����ʵ�ʲ����ڹ�ҵ�������� (����)��
| A��CO2ͨ������������Һ����Na2CO3 |
| B��H2��Cl2������HCl |
| C��Cl2ͨ�����ʯ��ˮ����Ư�� |
| D������������� |