��Ŀ����
����Ŀ����֪���������[(NH4)2SO4��FeSO4��6H2O]�׳�Ī���Σ���Ħ������Ϊ392 g/mol��������ˮ����100��~ 110 ��ʱ�ֽ⡣Ϊ̽���仯ѧ��ɣ�������ͬѧ���������ʵ�顣
��.̽��Ī���ξ������ʱ�ķֽ���
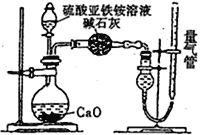
��1����ͬѧ�������ͼ��ʾ��װ�ý���ʵ�飬װ��C�пɹ۲쵽����������Һ��죬�ɴ˿�֪�ֽ��������_____________��

��2����ͬѧ��ΪĪ���ξ���ֽ�IJ����л�����SO3(g)��SO2(g)��N2(g)��Ϊ��֤����Ĵ��ڣ�������װ�ý���ʵ�飺

������ͬѧ��ʵ���У�װ���������ӵĺ���˳��ΪA��H��__________________________��G
��֤������SO3��ʵ��������_______________��
II.Ϊ����������林���,��ȡm g Ī������Ʒ�����500 mL��Һ���ס�����ͬѧ�������������ʵ�鷽����
������ȡ25.00mL�����������Һ��0.1000molL-1������K2Cr2O7��Һ�����ν��еζ���
�ҷ�����(ͨ��NH4+�ⶨ)���װ������ͼ��ʾ��ȡ25.00 mL��Ʒ��Һ���и�ʵ�顣
��1���ζ������У�����K2Cr2O7��ҺӦװ��_____________�ζ����С������е����ӷ���ʽΪ_________________________________��
��2���ҷ�����������������Լ���______������ĸ����
a.ˮ b.����NaHCO3��Һ c.CC14
��3���ҷ������ռ������岢�ָ������£�����ǰӦ���еIJ�����______________________________��
��4�������NH3(������Ϊ��״����)ΪVL������������林���Ϊ___________��(�ú�V��m��ʽ�ӱ�ʾ)
���𰸡� NH3 F��D��E F�г��ְ�ɫ���� ��ʽ Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O c �����ƶ������ܣ��ζ��ܣ���ʹ��������Һ����ƽ 175 V/m��100%��![]() ��100%
��100%
��������I.��1������Ī���ε���ɣ�Ī�������ȷֽ������ʹ��̪��Һ��������ΪNH3��
��2����ͬѧ��ΪĪ���ξ���ֽ�IJ����л�����SO3��g����SO2��g����N2��g������BaCl2����������Ļ��Һ����SO3��g������Ʒ����Һ����SO2��g����N2������ˮ����ˮ���ռ�������SO3�ܱ�ˮ��Һ���գ������ȼ���SO3��g�����ټ���SO2��g������NaOH��Һ��ȥSO2���������ˮ���ռ�N2��װ�õĺ�������˳��ΪA��H��F��D��E��G��
II.��1������K2Cr2O7��Һ����ǿ�����ԣ�Ӧװ����ʽ�ζ����С�Cr2O72-��Fe2+������Fe3+����������ԭ��Cr3+�����ݵ�ʧ�����غ㡢ԭ���غ�͵���غ���ƽ��
��2�������ҷ�����װ�ã������������Һ��CaO��Ӧ����NH3���ü�ʯ�ҳ�ȥNH3�е�H2O��g��������������ȡNH3�������ˮ�ͱ���NaHCO3��Һ������NH3��NH3������CCl4��������������Լ�ΪCCl4��
��3�����������������ж���ʵ��ʱ������Ҫ�����¶Ⱥ�ѹǿ������ʵ�����Ҫ�ָ������£�ʹ���������ѹǿ���ڴ���ѹ��
��4������N�غ������������淋���������һ����������������
I.��1��װ��B�еļ�ʯ�����շֽ�������������壬װ��C�з�̪��Һ��죬����Ī���ε���ɣ���Ī�������ȵķֽ��������NH3��
��2����ͬѧ��ΪĪ���ξ���ֽ�IJ����л�����SO3��g����SO2��g����N2��g������BaCl2����������Ļ��Һ����SO3��g������Ʒ����Һ����SO2��g����N2������ˮ����ˮ���ռ�������SO3�ܱ�ˮ��Һ���գ������ȼ���SO3��g�����ټ���SO2��g������NaOH��Һ��ȥSO2���������ˮ���ռ�N2��װ�õĺ�������˳��ΪA��H��F��D��E��G��
����������������װ�õĺ�������˳��ΪA��H��F��D��E��G��
��֤������SO3��ʵ�������ǣ�װ��F�г��ְ�ɫ������
II.��1������K2Cr2O7��Һ����ǿ�����ԣ�Ӧװ����ʽ�ζ����С�Cr2O72-��Fe2+������Fe3+����������ԭ��Cr3+��1molCr2O72-���뷴Ӧ�õ�6mol������1molFe2+���뷴Ӧʧȥ1mol���ӣ����ݵ�ʧ�����غ㡢ԭ���غ�͵���غ㣬�����е����ӷ���ʽΪCr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
��2�������ҷ�����װ�ã������������Һ��CaO��Ӧ����NH3���ü�ʯ�ҳ�ȥNH3�е�H2O��g��������������ȡNH3�������a�����NH3��������ˮ��ˮ������NH3��b��������NH3��������ˮ����ˮ��Һ�ʼ��ԣ�����NaHCO3��Һ����NH3��c����NH3������CCl4��������NH3��������������Լ�ΪCCl4����ѡc��
��3�����������������ж���ʵ��ʱ������Ҫ�����¶Ⱥ�ѹǿ������ʵ�����Ҫ�ָ������£�ʹ���������ѹǿ���ڴ���ѹ���ҷ������ռ������岢�ָ������£�����ǰӦ���еIJ����ǣ������ƶ������ܣ��ζ��ܣ���ʹ��������Һ����ƽ��
��4������N�غ㣬25.00mL��Ʒ��n[��NH4��2SO4��FeSO4��6H2O]=![]() n��NH3��=
n��NH3��=![]() mol��500mL��Һ��n[��NH4��2SO4��FeSO4��6H2O]=
mol��500mL��Һ��n[��NH4��2SO4��FeSO4��6H2O]=![]() mol
mol![]() =
=![]() mol��m[��NH4��2SO4��FeSO4��H2O]=
mol��m[��NH4��2SO4��FeSO4��H2O]=![]() mol
mol![]() 392g/mol=
392g/mol=![]() g������������林���Ϊ
g������������林���Ϊ![]() 100%=
100%=![]() 100%��
100%��

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�