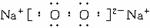��Ŀ����
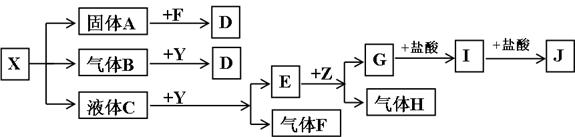
��ͼ�е����ʾ�Ϊ��ѧ��ѧ���������ʣ�����X��Y��ZΪ���ʣ�A��HΪ�����B��H��Ħ��������ͬ��E����Է���������D����Է���������16����һ�������£��������ת����ϵ��ͼ��ʾ��

�ش��������⣺
(1)Z�Ļ�ѧʽΪ_________________��G�Ļ�ѧʽΪ__________________��
(2)H�ĵ���ʽΪ_________________________________________________��
(3)д��X��B�ֱ��ˮ��Ӧ�Ļ�ѧ����ʽ��
X+H2O_________________________________________________________��
B+H2O_________________________________________________________��
(4)д��C+G��E�����ӷ�Ӧ����ʽ��______________________________��
(1)O2 SO3
(2)![]()
(3)2Na+2H2O====2HaOH+H2�� 2Na2O2+H2O====4NaO H+O2��
(4)SO3+2OH-====![]() +H2O
+H2O

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ