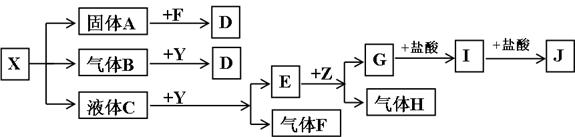
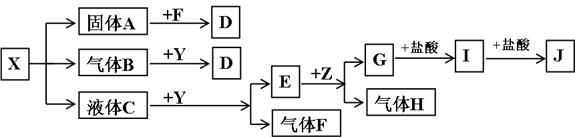
��Ŀ����
��ͼ���������ʾ�Ϊ��ѧ��ѧ�������ʣ�F��H�����嵥�ʣ�Z�ǹ���������ʣ�Y���������������Ӹ�����Ϊ2��1��������X��Y��A��D��E��G����ɫ��ӦΪ��ɫ��I�ǰ�ɫ������
��1��д��X��E��I�Ļ�ѧʽ��X__________��E__________��I__________��
��2��д��Y�ĵ���ʽ��__________��
��3������Y��˵����ȷ����__________��
A.Y�������� B.Y�ǹ�������
C.Y��ǿ����� D.Y�Ǽ���������
��4��д��B��Y��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
��5��X��G����Һ�ܷ�Ӧ�����ܷ�Ӧ��д����Ӧ�����ӷ���ʽ���粻�ܷ�Ӧ����˵������___________________________________________________________________��
��1��NaHSO3 NaOH Al��OH��3
��2��![]()
(3)ABC
(4)SO2+Na2O2====Na2SO4
(5)�ܷ�Ӧ��![]() +
+![]() +H2O====
+H2O====![]() +Al(OH)3��
+Al(OH)3��
��������X��Y��A��D��E��G����ɫ��ӦΪ��ɫ���ʿ���֪���Ǿ�����NaԪ�أ���Y���������������Ӹ�����Ϊ2��1�����Ʋ�YΪNa2O2��E+Z![]() G+H����ZΪ�������ʣ�HΪ���嵥�ʣ�����֪ZΪAl��EΪNaOH����X���ȷֽ�ù���A������B��Һ��C��֪X����ΪNaHCO3��NaHSO3���ɿ�ͼ��ϵ��֪XΪNaHSO3���Ӷ������Ƴ������ʡ���5������
G+H����ZΪ�������ʣ�HΪ���嵥�ʣ�����֪ZΪAl��EΪNaOH����X���ȷֽ�ù���A������B��Һ��C��֪X����ΪNaHCO3��NaHSO3���ɿ�ͼ��ϵ��֪XΪNaHSO3���Ӷ������Ƴ������ʡ���5������![]() �ĵ���̶ȴ���Al��OH��3�ĵ���̶ȣ���
�ĵ���̶ȴ���Al��OH��3�ĵ���̶ȣ���![]() +
+![]() +H2O====
+H2O====![]() +Al��OH��3����
+Al��OH��3����

 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�