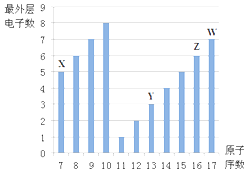
��Ŀ����
����Ŀ�������Ļ�ѧ������������ơ���ش��������⣺
(1)�����Ļ�ѧ���ʱȽ��ȶ���ͨ����������ᡢ�________�ȶ�����Ӧ����������ȼ�ա���������������±�ط���__________��
(2)�ӷ��ӽṹ�ĽǶȣ����������������ƻ�ѧ���ʵ�ԭ��___________________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ(�л���д�ṹ��ʽ)��
�ٱ�����ȫȼ��_______________________________________________��
��������ȫȼ�յ�ͨʽ����ʽ _____________________________________________��
����������������ȡ����Ӧ(ֻҪ��дһ��ȡ��) ___________________________________��
������±����Ӧ(��X2��ʾ)��ͨʽ����ʽ _________________________________________��
���𰸡� ǿ������ ȡ����Ӧ ���������е�̼ԭ����̼̼������ϳ���״��ʣ��ۼ�������ԭ�ӽ�ϣ�ʹÿ��̼ԭ�ӵĻ��ϼ۶��ﵽ�����͡� CH3CH2CH3��5O2![]() 3CO2��4H2O CnH2n��2��
3CO2��4H2O CnH2n��2��![]() O2
O2![]() nCO2��(n��1)H2O CH3CH3��Cl2
nCO2��(n��1)H2O CH3CH3��Cl2![]() CH3CH2Cl��HCl CnH2n��2��X2
CH3CH2Cl��HCl CnH2n��2��X2![]() CnH2n��1X��HX( CnH2n��1X����X2��������ȡ����Ӧ)
CnH2n��1X��HX( CnH2n��1X����X2��������ȡ����Ӧ)
������������Ϊ����������ͨʽΪCnH2n��2����ѧ���ʱȽ��ȶ������������ᡢ�ǿ����������Ӧ����������ȼʱ��ȼ�գ��ڹ��������£�����������������±�ص��ʷ���ȡ����Ӧ���ݴ˷��������
(1)�����Ļ�ѧ���ʱȽ��ȶ���ͨ����������ᡢ�ǿ�������ȶ�����Ӧ����������ȼ�ա���������������±�ط���ȡ����Ӧ����ȷ�𰸣�ǿ�������� ȡ����Ӧ��
(2) ���������е�̼ԭ����̼̼������ϳ���״��ʣ��ۼ�������ԭ�ӽ�ϣ�ʹÿ��̼ԭ�ӵĻ��ϼ۶��ﵽ�����͡������������������ƻ�ѧ���ʣ���ȷ�𰸣����������е�̼ԭ����̼̼������ϳ���״��ʣ��ۼ�������ԭ�ӽ�ϣ�ʹÿ��̼ԭ�ӵĻ��ϼ۶��ﵽ�����͡���
(3)�ٱ�����ȫȼ�����ɶ�����̼��ˮ��CH3CH2CH3��5O2![]() 3CO2��4H2O ����ȷ����CH3CH2CH3��5O2
3CO2��4H2O ����ȷ����CH3CH2CH3��5O2![]() 3CO2��4H2O��
3CO2��4H2O��
������ͨʽΪCnH2n��2����ȫȼ�����ɶ�����̼��ˮ��CnH2n��2��![]() O2
O2![]() nCO2��(n��1)H2O����ȷ�𰸣�CnH2n��2��
nCO2��(n��1)H2O����ȷ�𰸣�CnH2n��2��![]() O2
O2![]() nCO2��(n��1)H2O��
nCO2��(n��1)H2O��
����������������ȡ����Ӧ��������������Ȼ��⣺CH3CH3��Cl2![]() CH3CH2Cl��HCl����ȷ�𰸣�CH3CH3��Cl2
CH3CH2Cl��HCl����ȷ�𰸣�CH3CH3��Cl2![]() CH3CH2Cl��HCl��
CH3CH2Cl��HCl��
������ͨʽΪCnH2n��2������±����Ӧ(��X2��ʾ)��ͨʽ����ʽ��CnH2n��2��X2![]() CnH2n��1X��HX( CnH2n��1X����X2��������ȡ����Ӧ)����ȷ�𰸣�CnH2n��2��X2
CnH2n��1X��HX( CnH2n��1X����X2��������ȡ����Ӧ)����ȷ�𰸣�CnH2n��2��X2![]() CnH2n��1X��HX( CnH2n��1X����X2��������ȡ����Ӧ)��
CnH2n��1X��HX( CnH2n��1X����X2��������ȡ����Ӧ)��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ�������������ᡢ���ص����ʵ���Ҫԭ�ϡ�
(1)�����������ǹ�ҵ���������Ҫ��������֪:
2NO(g)+3H2(g) ![]() 2NH3(g)+02(g) ��H1=-272.9kJ/mol
2NH3(g)+02(g) ��H1=-272.9kJ/mol
2H2(g)+02(g)![]() 2H2O(g) ��H2=-483.6kJ/mol
2H2O(g) ��H2=-483.6kJ/mol
��4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g) ��H3=__________
4NO(g)+6H2O(g) ��H3=__________
(2)�����ܱ������н��кϳ�֪��Ӧ:N2(g)+3H2(g)![]() 2NH3(g) ��H4==-92.4kJ/mol���仯ѧƽ�ⳣ��(K)���¶ȵĹ�ϵ���±�:
2NH3(g) ��H4==-92.4kJ/mol���仯ѧƽ�ⳣ��(K)���¶ȵĹ�ϵ���±�:
�¶�/K | 298 | 398 | 498 | �� |
ƽ�ⳣ��(K) | 4.1��106 | K1 | K2 | �� |
K1____K2(�>����<��),���������_____________��
(3)�ϳ����г���10molN2��40molH2�ϳɰ���һ���¶�(T)��ƽ�������а��������������ѹǿ(p)�Ĺ�ϵ��ͼ1��ʾ��

������˵����ȷ����______(����ĸ)��
A.��ͼ1��֪������ϵѹǿ(p)�������������ڻ�������е��������
B.��ͼ1��T=500�棬���¶�Ϊ450��ʱ��Ӧ��������b
C.��ҵ�ϲ���500���¶ȵ���ҪĿ������ߵ�����ת����
D.��3v��(H2)=2v��(NH3)ʱ����Ӧ�ﵽƽ��״̬
�ڵ��¶�ΪT���������������Ϊ25%ʱ��N2��ת����Ϊ__________��
(4)��ҵ����NH3��������ʱ����NH3��O2�������1��2���ͨ��ij�ض��������ܱ������н��з�Ӧ���������ʲ�����罻�������õ���Һ�����ʵ���������Ϊ_______��
(5)��̼��[![]() ]�Ժϳ����صķ�Ӧ��2NH3(g)+CO2(g)
]�Ժϳ����صķ�Ӧ��2NH3(g)+CO2(g)![]() CO(NH2)2(g)+H2O(g)��Ӱ�졣T��ʱ����2L�ĺ����ܱ������У������ʵ���֮��Ϊ3mol��NH3��CO2�Բ�ͬ�İ�̼�Ƚ��з�Ӧ�������ͼ2��ʾ��a��b�ֱ��ʾCO2��NH3��ת���ʣ�c��ʾƽ����ϵ�����ص������������
CO(NH2)2(g)+H2O(g)��Ӱ�졣T��ʱ����2L�ĺ����ܱ������У������ʵ���֮��Ϊ3mol��NH3��CO2�Բ�ͬ�İ�̼�Ƚ��з�Ӧ�������ͼ2��ʾ��a��b�ֱ��ʾCO2��NH3��ת���ʣ�c��ʾƽ����ϵ�����ص������������![]() =_______ʱ�����صIJ�����������·�Ӧ��ƽ�ⳣ��K=________��
=_______ʱ�����صIJ�����������·�Ӧ��ƽ�ⳣ��K=________��