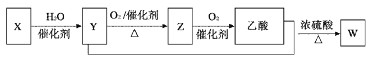
��Ŀ����
����Ŀ��T ��ʱ����2.0 L�����ܱ������г���1.0 mol PCl5����ӦPCl5(g) ![]() PCl3(g)��Cl2(g)����һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ�IJ���������ͼ��ʾ������˵����ȷ����
PCl3(g)��Cl2(g)����һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ�IJ���������ͼ��ʾ������˵����ȷ����
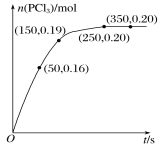
A.��Ӧ��ǰ50 s��ƽ������v(PCl3)=0.0032 mol��L-1��s-1
B.��ͬ�¶��£���ʼʱ�������г���2.0 mol PCl3��2.0 mol Cl2���ﵽƽ��ʱ��PCl3��ת����С��80%
C.T ��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.025
D.��ͬ�¶��£���ʼʱ�������г���1.0 mol PCl5��0.20 mol PCl3 ��0.20 mol Cl2����Ӧ�ﵽƽ��ǰv����v��
���𰸡�CD
��������
A����ͼ����ȡ������Ϣ��50 sʱ��n(PCl3)=0.16mol��c(PCl3)=0.08mol/L��v(PCl3)=![]() =0.0016mol��L-1��s-1��A����
=0.0016mol��L-1��s-1��A����
B�� PCl5(g) ![]() PCl3(g)��Cl2(g)
PCl3(g)��Cl2(g)
��ʼ�� 0.5mol/L 0 0
�仯�� 0.1mol/L 0.1mol/L 0.1mol/L
ƽ���� 0.4mol/L 0.1mol/L 0.1mol/L
��(PCl5)=![]() ����ͬ�¶��£���ʼʱ�������г���1.0 mol PCl3��1.0 mol Cl2���ﵽƽ��ʱ��PCl3��ת����Ϊ80%����ͬ�¶��£���ʼʱ�������г���1.0 mol PCl3��1.0 mol Cl2�����൱��ԭƽ����ϵ��ѹ��ʹ���������Ϊԭ����һ�룬ƽ�������ƶ����ﵽƽ��ʱ��PCl3��ת���ʴ���80%��B����
����ͬ�¶��£���ʼʱ�������г���1.0 mol PCl3��1.0 mol Cl2���ﵽƽ��ʱ��PCl3��ת����Ϊ80%����ͬ�¶��£���ʼʱ�������г���1.0 mol PCl3��1.0 mol Cl2�����൱��ԭƽ����ϵ��ѹ��ʹ���������Ϊԭ����һ�룬ƽ�������ƶ����ﵽƽ��ʱ��PCl3��ת���ʴ���80%��B����
C. T ��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=![]() =0.025��C��ȷ��
=0.025��C��ȷ��
D. ��ͬ�¶��£���ʼʱ�������г���1.0 mol PCl5��0.20 mol PCl3 ��0.20 mol Cl2��Ũ����Q=![]() =0.02<0.025������ƽ�������ƶ�����Ӧ�ﵽƽ��ǰv����v����D��ȷ��
=0.02<0.025������ƽ�������ƶ�����Ӧ�ﵽƽ��ǰv����v����D��ȷ��
��ѡCD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���������ƣ�NaN3����������ȫ��������������巢����ԭ�ϡ������ǹ�ҵˮ���·��Ʊ��������ƵĹ������̣�

�������ϣ�
��ˮ���£�N2H4��H2O���ж��Ҳ��ȶ�������ǿ��ԭ�Ժ�ǿ���ԡ�
���й����ʵ������������±���
���� | �۵�� | �е�� | �ܽ��� |
CH3OH | -97 | 64.7 | ��ˮ���� |
ˮ����(N2H4��H2O) | -40 | 118.5 | ��ˮ�������ܣ����������� |
���������(CH3ONO) | -17 | -12 | �����Ҵ������� |
��������(NaN3) | 275 (410�ֽ�) | ���� | ������ˮ�����ڴ��� ���������� |
��1���ϳ�ˮ���¡�
ʵ���Һϳ�ˮ����װ����ͼ��ʾ��NaClO������Һ������CO(NH2)2ˮ��Һ��40�����·�Ӧһ��ʱ�����Ѹ��������110�������Ӧ�����Ƶ�ˮ���¡�
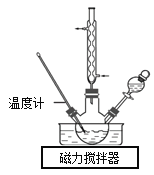
����ȡN2H4��H2O�����ӷ���ʽΪ_________��
��ʵ����ͨ����Һ©���μӵ���Һ��_________��������_________��
��2��ˮ���·��Ʊ��������ơ�
��ʵ����ģ������̲�����Ʊ��������Ƶķ�Ӧԭ��Ϊ��N2H4��H2O(aq)+CH3ONO(g)+NaOH(aq)=NaN3(aq)+CH3OH(aq)+3H2O(l) ��H��0�� �о������÷�Ӧ��20�����ҷ�Ӧ��ѡ���Ժ�ת������ߣ�ʵ��ʱ���Բ�ȡ�Ĵ�ʩ��_________��
�ڲ�������CH3OH��ʵ���������Ϊ________��
���������B��Һ��õ������ƣ�NaN3����Ʒ��ʵ�鷽����_________[ʵ���пɹ�ѡ����Լ��У��Ҵ������ѣ���������������ʹ�õ������У�����©������ո�����]��
����Ŀ����3��2L���ܱ������У�����ͬ���¶��¡�ʹ����ͬ�Ĵ����ֱ���з�Ӧ��3H2(g)+N2(g)![]() 2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ�й��������£�
2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ�й��������£�
���� | �� | �� | �� |
��Ӧ���Ͷ���� | 3molH2��2molN2 | 6molH2��4molN2 | 2molNH3 |
�ﵽƽ���ʱ��/min | 5 | 8 | |
ƽ��ʱN2��Ũ��/mol��L-1 | c1 | 1.5 | |
NH3��������� | ��1 | ��3 | |
���������ܶ�/g��L-1 | ��1 | ��2 |
����˵����ȷ���ǣ� ��
A.2c1<1.5B.��1=��2
C.��1=2��3D.�ڸ��¶��¼������з�Ӧ��ƽ�ⳣ��K=![]()