��Ŀ����
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ���£�

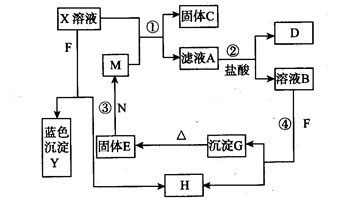
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫҺ�塣��ش��������⣺
��1��д��D��Һ�����ˮ�пɵõ����ɫҺ������ӷ���ʽ��________________����Һ����е�������___________������д��ţ�
�ٹ���ͨ����Һ��ʱ�γɹ����ġ�ͨ·����
�ڲ���缫ֱͨ�������һ������Һ����ɫ���
�����Һ���м�����������Һ������������
�ܽ���Һ����ȡ����ɡ����պ������������ɡ�
��2��A��B��H�Ļ�ѧʽ��A_______________B________________H_______________��
��3���� H2O2���ӵĵ���ʽ��______________
�� д��C��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ___________________��
��4����C��Һ������C�����ʵ�����Na2O2��ǡ��ʹCת��ΪF��д���÷�Ӧ�����ӷ���ʽ��

��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫҺ�塣��ش��������⣺
��1��д��D��Һ�����ˮ�пɵõ����ɫҺ������ӷ���ʽ��________________����Һ����е�������___________������д��ţ�
�ٹ���ͨ����Һ��ʱ�γɹ����ġ�ͨ·����
�ڲ���缫ֱͨ�������һ������Һ����ɫ���
�����Һ���м�����������Һ������������
�ܽ���Һ����ȡ����ɡ����պ������������ɡ�
��2��A��B��H�Ļ�ѧʽ��A_______________B________________H_______________��
��3���� H2O2���ӵĵ���ʽ��______________
�� д��C��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ___________________��
��4����C��Һ������C�����ʵ�����Na2O2��ǡ��ʹCת��ΪF��д���÷�Ӧ�����ӷ���ʽ��
��1��3Fe3+��3H2O��Fe(OH)3�����壩��3H+ ��2�֣� �٢ڢ� ��2�֣�ѡһ�����÷֣�ѡ������1�֣�ѡ3����2�֣�
��2��A��Fe �� B��FeS�� H��H2SO4��ϡ�� ����1�ֹ�3�֣�
��3��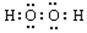 ��1�֣�
��1�֣�
2Fe2+��2H2O2��2H+��2Fe3+��2H2O ��2�֣�
��4��4Fe2+��4 Na2O2��6H2O��4 Fe(OH)3����O2����8Na+��2��
��2��A��Fe �� B��FeS�� H��H2SO4��ϡ�� ����1�ֹ�3�֣�
��3��
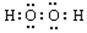 ��1�֣�
��1�֣�2Fe2+��2H2O2��2H+��2Fe3+��2H2O ��2�֣�
��4��4Fe2+��4 Na2O2��6H2O��4 Fe(OH)3����O2����8Na+��2��
��

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
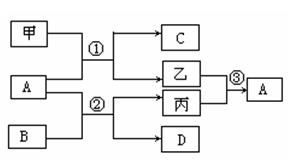


 A)ʱ��AΪ�� ��
A)ʱ��AΪ�� ��