��Ŀ����
(12��)�±�ΪԪ�����ڱ���һ���֣���Ҫ����ɸ�С�⡣
С��1:��ѧ��������õ�Ԫ�� ����Ԫ��ţ���ͬ�����ǽ�������ǿ��Ԫ���� ��������ǿ�ĵ�����ˮ��Ӧ�����ӷ���ʽΪ
С��2:�٢ۢ�����Ԫ�ص����������ˮ�����У�������ǿ��
С��3:�٢ۢ�����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ
С��4:ijԪ�ص�����������ˮ����������ᷴӦ�����κ�ˮ������Ӧ��������ˮ����Ԫ��Ϊ ���������и�Ԫ�صĻ��ϼ�Ϊ ����Ԫ�ص��������������ᷴӦ�Ļ�ѧ����ʽΪ ���Ԫ�غ͢��Ԫ����ɵĻ�������Һ�У������μ�NaOH������������Ϊ
 | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 2 | | | | �� | | �� | �� | |
| 3 | �� | �� | �� | | | | �� | �� |
| 4 | �� | �� | | | | | �� | |
С��2:�٢ۢ�����Ԫ�ص����������ˮ�����У�������ǿ��
С��3:�٢ۢ�����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ
С��4:ijԪ�ص�����������ˮ����������ᷴӦ�����κ�ˮ������Ӧ��������ˮ����Ԫ��Ϊ ���������и�Ԫ�صĻ��ϼ�Ϊ ����Ԫ�ص��������������ᷴӦ�Ļ�ѧ����ʽΪ ���Ԫ�غ͢��Ԫ����ɵĻ�������Һ�У������μ�NaOH������������Ϊ
��1:�� �� 2K + 2H2O ==2K + + 2OH�� + H2
��2: ��
С��3:�٢ۢ�
С��4:�� +3 AI2O3+6HCI==2AICI3+3H2O �����������Ƶĵ��룬��ɫ���������ӣ����ﵽ���������٣������ȫ��ʧ���õ�������Һ
����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ��е�λ�ÿ��жϣ��١��Ϸֱ�ΪNa��K��Mg��Ca��Al��C��O��Cl��Br��Ar��F��
��1��ϡ������Ԫ�ص���������ã��ǽ�������ǿ����F����������ǿ��K��
��2��������Խǿ������������ˮ����ļ��Ծ�Խǿ��ͬ����Ԫ����������ԭ�Ӱ뾶��С�������������������������Ƶļ�����ǿ��
��3���٢ۢ�����ͬһ���ڣ�����ԭ�Ӱ뾶��С˳��Ϊ�٢ۢ�
��4�����������Ⱥ��ᷴӦ��Ҳ��ǿ�Ӧ�����κ�ˮ�������ڵڢ�A�����ϼ��ǣ�3�ۡ�
��1��ϡ������Ԫ�ص���������ã��ǽ�������ǿ����F����������ǿ��K��
��2��������Խǿ������������ˮ����ļ��Ծ�Խǿ��ͬ����Ԫ����������ԭ�Ӱ뾶��С�������������������������Ƶļ�����ǿ��
��3���٢ۢ�����ͬһ���ڣ�����ԭ�Ӱ뾶��С˳��Ϊ�٢ۢ�
��4�����������Ⱥ��ᷴӦ��Ҳ��ǿ�Ӧ�����κ�ˮ�������ڵڢ�A�����ϼ��ǣ�3�ۡ�

��ϰ��ϵ�д�
�����Ŀ
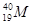 ��
��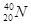 ��
��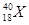 ��
�� ����
����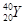 2����
2����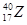 ������������Ԫ�ص�����Ϊ
������������Ԫ�ص�����Ϊ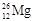 ��˵���У�����ȷ����
��˵���У�����ȷ����