��Ŀ����
��13�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⡣
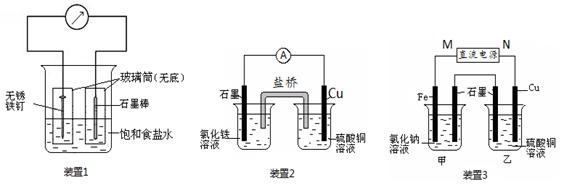
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ�������������IJ���Ͳ�ڵ���K3[Fe(CN)6] ��Һ�����ɹ۲쵽������������Һ����ɫ�������������� ��� ��������ԭ������ �����̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣����ձ��е��뼸�η�̪��Һ���۲쵽ʯī�缫�������ȱ�졣
�� ��Դ��M��Ϊ ��������������������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�Ļ�ѧ����ʽΪ ��
�� ֹͣ��⣬ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0.64 g�����ձ��в����������״�������Ϊ mL ��
��13�֣���1��������1�֣��� O2+ 4e��+ 2H2O = 4OH����2�֣�
��2������1�֣��� 2Fe3�� + Cu = 2Fe2�� + Cu2�� ��2�֣�
��3���� ����1�֣��� Fe -2e��= Fe2����2�֣�
�� 2CuSO4+ 2H2O 2Cu + O2�� + 2H2SO4 ��2�֣� �� 224 ��2�֣�
2Cu + O2�� + 2H2SO4 ��2�֣� �� 224 ��2�֣�
��������
�����������1��װ��1Ϊ���ĵ绯ѧ��ʴ��������������Һ����ɫ����������������������Ϊ������̼Ϊ�������۲쵽̼����������Һ��죬˵����̼���������õ�������OH-���ӣ���Ӧ�ĵ缫��ӦʽΪO2+ 4e��+ 2H2O = 4OH����
��2��װ��2Ϊԭ��أ�����ΪCu��ͭʧȥ���ӣ�����ͭ���Ӷ��ܽ⡣ʯī����������Һ�е������ӵõ����������������ӣ������ܵ����ӷ���ʽ��2Fe3�� + Cu = 2Fe2�� + Cu2����
��3���ٷ�Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽ʯī�缫�������ȱ�죬˵����ʯī�缫������OH-���ӣ���ʯī����������������������M�ǵ�Դ�������������缫�ķ���ʽ��Fe -2e��= Fe2����
�����ձ��������ͭ��Һ��ʯīΪ������ͭ�����������Ե����ܷ���ʽ��2CuSO4��2H2O 2Cu �� O2���� 2H2SO4��
2Cu �� O2���� 2H2SO4��
��ȡ��Cu�缫��ϴ�ӡ�����������缫����0.64g��������Cu�����ʵ���Ϊ0.64g ��64g/mol ��0.01mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol��2��0.02mol
���ݼ��ձ���������ĵ缫��Ӧ��֪
2H��+2e����H2��
2mol 22.4L
0.02mol V
����V��0.224L��
���㣺����绯ѧԭ�����ۺ�Ӧ�á������ĸ�ʴ�ͷ������缫��Ӧʽ����д���缫���Ƶ��ж��Լ��йؼ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ���������У����ؿ���ѧ���������⡢������������������ʱҪע��缫���жϺ͵缫��Ӧ����д��ע�����·�и��缫ת�Ƶĵ�����Ŀ��ȣ����÷�Ӧ�ķ���ʽ���㼴�ɡ�

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
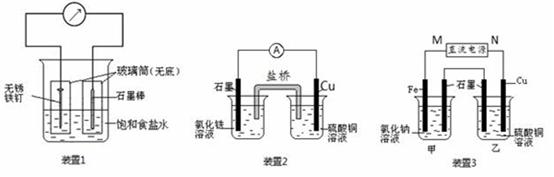
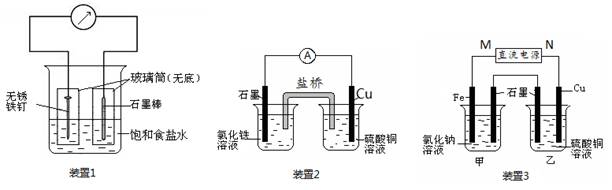 ��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��