��Ŀ����
����Ŀ����������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42����NO3����Cl�� ��ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ������������Һ����Ʋ���������µ�ʵ�飺

��֪��3NO3��+ 8Al + 5OH�� + 2H2O![]() 3NH3 + 8AlO2��
3NH3 + 8AlO2��
�������ϵ�ʵ�����������ͬѧ�ó��Ľ�������ȷ����
�����п϶�����NH4+��Mg2+��SO42����NO3��
������һ������Al3+
�����п��ܴ���Na+��Cl��
�������п��ܴ���NaNO3 ��NH4Cl��MgSO4
���𰸡�B
��������
����Al3+��Ba(OH)2��Һ������AlO2-��ͨ��CO2������Al(OH)3�������2�У�����H+�ֱ��ܽ⡣�ʿ��������������ܴ��ڡ�

����Ŀ����֪��KClO3��6HCl(Ũ)=KCl��3Cl2����3H2O����ͼ��ʾ���������Լ��ֱ�����������е���Ӧλ�ã�ʵ��ʱ��Ũ�������KClO3�����ϣ����ñ�����Ǻá��±�����ʵ������ó��Ľ�����ȫ��ȷ����(����)
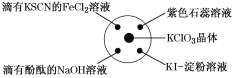
ѡ�� | ʵ������ | ���� |
A | ����KSCN��FeCl2��Һ��� | Cl2���л�ԭ�� |
B | ���з�̪��NaOH��Һ��ɫ | Cl2�������� |
C | ��ɫʯ����Һ�ȱ�����ɫ | Cl2����Ư���� |
D | KI������Һ�����ɫ | Cl2���������� |
A.AB.BC.CD.D