��Ŀ����
�����ʵ���Ҫ�ɷ��ǻ���ʯ[Ca10��PO4��6��OH��2]��ˮ���ܼ����ܽ�Ca10��PO4��6��OH��2��s��?10Ca2+��aq��+6PO43-��aq��+2OH-��aq������֪25��ʱKsp[Ca10��PO4��6��OH��2]=2.35��10-59��Ksp[Ca10��PO4��6F2]=7.1��10-61��Ksp[CaCO3]=5��10-9��Ksp[CaF2]=4��10-11������˵������ȷ�ǣ�������
| A����ǻ�ڲ���ʳ��ᷢ��ʹ��ǻ�����ԣ����Է���˯ǰӦ��Ҫ���� |
| B������ˮ�з�Ԫ�غ����ϵ͵ĵ���ʹ�ú����������Ч��ֹȣ�� |
| C��25��ʱ����CaCO3��Һ�ͱ���CaF2��Һ��ȣ�����c��Ca2+���ϴ� |
| D��25��ʱ����CaCO3����Һ�м���NaF��Һ��CaCO3������ת��ΪCaF2 |
A����ǻ�ڲ���ʳ��ᷢ��ʹ��ǻ�����ԣ������ʵ���Ҫ�ɷ��ǻ���ʯ�ܽ�Ca10��PO4��6��OH��2��s��?10Ca2+��aq��+6PO43-��aq��+2OH-��aq�����ɵ��������������������ӷ�Ӧ��ƽ�����ܽⷽ���ƶ��������������𣬹�A��ȷ��
B��ʹ�ú��������ʹ[Ca10��PO4��6��OH��2]ת��Ϊ�����ܵ�[Ca10��PO4��6F2]������Ч��ֹȣ�ݣ���B��ȷ��
C.25��ʱ����CaCO3��Һ�ͱ���CaF2��Һ��ȣ�CaCO3��Һc��Ca2+��=
mol/L��CaF2��Һc��Ca2+��=
mol/L����CaF2��Һ��c��Ca2+���ϴ�C��ȷ��
D����CaCO3����Һ�м���NaF��Һ��c��Ca2+����c2��F-����Ksp[CaF2]ʱ��������CaF2����D����
��ѡD��
B��ʹ�ú��������ʹ[Ca10��PO4��6��OH��2]ת��Ϊ�����ܵ�[Ca10��PO4��6F2]������Ч��ֹȣ�ݣ���B��ȷ��
C.25��ʱ����CaCO3��Һ�ͱ���CaF2��Һ��ȣ�CaCO3��Һc��Ca2+��=
| 5��10-9 |
| 3 | 4��10-11 |
D����CaCO3����Һ�м���NaF��Һ��c��Ca2+����c2��F-����Ksp[CaF2]ʱ��������CaF2����D����
��ѡD��

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ
�����ʵ���Ҫ�ɷ��ǻ���ʯ[Ca10(PO4)6(OH)2]��ˮ���ܼ����ܽ�Ca10(PO4)6(OH)2(s)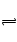 10Ca2+(aq)+6PO43-(aq)+2OH-(aq)����֪25��ʱKsp[Ca10(PO4)6(OH)2]="2.35" ��10-59�� Ksp[Ca10(PO4)6F2]=7.1�� 10-61��Ksp[CaCO3]="5" ��10-9��Ksp[CaF2]=4��10-11������˵������ȷ�ǣ� ��
10Ca2+(aq)+6PO43-(aq)+2OH-(aq)����֪25��ʱKsp[Ca10(PO4)6(OH)2]="2.35" ��10-59�� Ksp[Ca10(PO4)6F2]=7.1�� 10-61��Ksp[CaCO3]="5" ��10-9��Ksp[CaF2]=4��10-11������˵������ȷ�ǣ� ��
| A����ǻ�ڲ���ʳ��ᷢ��ʹ��ǻ�����ԣ����Է���˯ǰӦ��Ҫ���� |
| B������ˮ�з�Ԫ�غ����ϵ͵ĵ���ʹ�ú����������Ч��ֹȣ�� |
| C��25��ʱ����CaCO3��Һ�ͱ���CaF2��Һ��ȣ�����c(Ca2��)�ϴ� |
| D��25��ʱ����CaCO3����Һ�м���NaF��Һ��CaCO3������ת��ΪCaF2 |
 10Ca2+(aq)+6PO43-(aq)+2OH-(aq)����֪25��ʱKsp[Ca10(PO4)6(OH)2]=2.35
10Ca2+(aq)+6PO43-(aq)+2OH-(aq)����֪25��ʱKsp[Ca10(PO4)6(OH)2]=2.35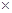 10-59�� Ksp[Ca10(PO4)6F2]=7.1
10-59�� Ksp[Ca10(PO4)6F2]=7.1