��Ŀ����
����Ŀ��������֣�ֻ��þ������̼����Ԫ�صľ��徹ȻҲ���г����ԡ�����������Ԫ�ض��dz���Ԫ�أ��Ӷ�����㷺��ע��
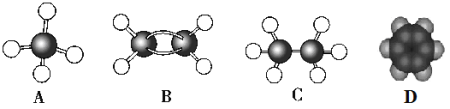
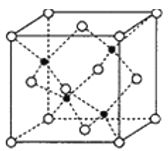
(1)�����ͳ��������һ��������ͼ��ʾ����þ���Ļ�ѧʽ��__________������Ԫ�����ڱ��е�λ����______��Ni2+�ļ۵�����_________�ֲ�ͬ�˶�״̬��
(2)500-600��ʱ��BeCl2��˫�۷��Ӵ��ڵ�BeCl2�ĽṹʽΪ________________________��
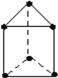
(3)�Ⱥ�ɫ�����ʻ��ܵ��۵�Ϊ52�棬����ʽΪCo2(CO)8����һ����Ҫ�����������������ڶ����л��ܼ����þ�������______���壬������______��������д��ڵ�������������___________(����)��
A�������� B�����Ӽ� C�����ۼ� D����λ�� E����� F�����»���
(4)����Ѫ��ķ��ӽṹ��ͼ��ʾ��������̼ԭ�ӵĹ���ӻ�����Ϊ_______���Ʋ⿹��Ѫ����ˮ�е��ܽ��ԣ�________(����������ˮ������������ˮ��)��
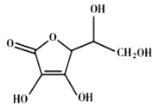
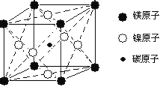
(5)����Ԫ��Zn��Ԫ��Se�γɵ�ij����������������ϵ���侧���ṹ��ͼ��ʾ������Ϊ( ![]() ΪSe��
ΪSe��![]() ΪZn)��Zn����λ��Ϊ______���þ�����ܶ�Ϊ
ΪZn)��Zn����λ��Ϊ______���þ�����ܶ�Ϊ![]() g/cm3����Zn��Se���ļ���Ϊ______nm��
g/cm3����Zn��Se���ļ���Ϊ______nm��
���𰸡�MgNi3C �������ڵڢ��� 8 ![]() ���� CO ACDF sp3��sp2 ������ˮ 4
���� CO ACDF sp3��sp2 ������ˮ 4 ![]()
��������
(1)�ڸ����ͳ��������һ�������У�����þԭ�ӵĸ���Ϊ![]() =1��������ԭ�ӵĸ���Ϊ
=1��������ԭ�ӵĸ���Ϊ![]() =3������̼ԭ�ӵĸ���Ϊ1����þ���Ļ�ѧʽ��MgNi3C����̬��ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d84s2��������Ԫ�����ڱ��е�λ���ǵ������ڵ����壬Ni2+�ļ۵����Ų�ʽΪ3d8����8�ֲ�ͬ�˶�״̬����Ϊ��MgNi3C���������ڵ����壻8��
=3������̼ԭ�ӵĸ���Ϊ1����þ���Ļ�ѧʽ��MgNi3C����̬��ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d84s2��������Ԫ�����ڱ��е�λ���ǵ������ڵ����壬Ni2+�ļ۵����Ų�ʽΪ3d8����8�ֲ�ͬ�˶�״̬����Ϊ��MgNi3C���������ڵ����壻8��
(2)500-600��ʱ��BeCl2��˫�۷��Ӵ��ڣ��������ʽΪBe2Cl4���ṹʽ��ÿ��Beԭ��Ӧ�γ�2����λ�������ܴ�8�����ȶ��ṹ������BeCl2�ĽṹʽΪ![]() ����Ϊ��
������![]() ��
��
(3)�Ⱥ�ɫ�����ʻ��ܵ��۵�Ϊ52��������ʽΪCo2(CO)8�������ڶ����л��ܼ�����þ������ڷ��Ӿ��壬������CO���������ĽṹʽΪ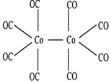 ���ɴ˿�ȷ�����ڵ������������н�����(��ԭ�Ӽ�)�����ۼ�(C��Oԭ�Ӽ�)����λ��(Co��Cԭ�Ӽ�)�����»���(����֮��)����ѡACDF����Ϊ�����ӣ�CO��ACDF��
���ɴ˿�ȷ�����ڵ������������н�����(��ԭ�Ӽ�)�����ۼ�(C��Oԭ�Ӽ�)����λ��(Co��Cԭ�Ӽ�)�����»���(����֮��)����ѡACDF����Ϊ�����ӣ�CO��ACDF��
(4)�ɿ���Ѫ��ķ��ӽṹ����ȷ����������Ԫ���ұߵ�3��̼ԭ�Ӽ۲���Ӷ���Ϊ4����Ԫ����ߵ�3��̼ԭ�ӵļ۲���Ӷ���Ϊ3������̼ԭ�ӵĹ���ӻ�����Ϊsp3��sp2������Ѫ������к���4��ˮ���Ի��ţ�������ˮ�е��ܽ��ԣ�������ˮ����Ϊ��sp3��sp2��������ˮ��
(5)��ͼ�п�֪��ÿ��Znԭ����Χ��4��Seԭ�ӣ��Ӷ�ȷ��Zn����λ��Ϊ4���þ�����ܶ�Ϊ![]() g/cm3���ڸþ����У�����4��ZnSe����������ı߳�(��Ϊa nm)Ϊa=
g/cm3���ڸþ����У�����4��ZnSe����������ı߳�(��Ϊa nm)Ϊa=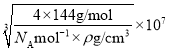 nm/cm=
nm/cm=![]() nm���ھ����й���1��ֱ�������Σ��ڴ�ֱ���������У�1��ֱ�DZ߳���������߳����ķ�֮һ��1��ֱ�DZ߳��������ζԽ��ߵ��ķ�֮һ��1��б�߳���Zn��Se���ļ����� ��Zn��Se���ļ���Ϊ
nm���ھ����й���1��ֱ�������Σ��ڴ�ֱ���������У�1��ֱ�DZ߳���������߳����ķ�֮һ��1��ֱ�DZ߳��������ζԽ��ߵ��ķ�֮һ��1��б�߳���Zn��Se���ļ����� ��Zn��Se���ļ���Ϊ![]() =
=![]() =
=![]() nm������4��
nm������4��![]() ��
��

 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�