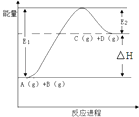
��Ŀ����
����Ŀ����1��ʵ��������ʢ�ż����Լ����Լ�ƿ����ĥ�ڲ�������ԭ�����������ӷ���ʽ��ʾ����______________________________�������Լ�ƿ����ʢ��������ԭ�������û�ѧ����ʽ��ʾ����_____________________________________��
��2��ʵ�����е�Na2SiO3��Һ���ڷ��ã�ƿ����ְ�ɫ���������γɳ��������ӷ���ʽ��___________________________��ȡƿ�е��ϲ���Һ����ϡ���ᣬ�۲쵽�������ݲ������г������ɣ������ӷ���ʽ�ֱ�Ϊ_____________________________________����Na2SiO3��Һ���ݹ���������ȼ�գ�����Na2SiO3����;����___________________��ԭ�ϡ�
��3��ij��Һ����NH4+��Mg2+��Fe2+��Fe3+��Al3+�������ӣ��������м������������������Һ���Ȳ����裬�ټ���������ᣬ��Һ�д������ٵ���������__________���������ӵ���������___________��
A. NH4+ B. Mg2+ C. Fe2+ D. Al3+ E. Fe3+
��4��������ͬ��H216O��D216O����������֮��Ϊ _______________�� ������֮��Ϊ____________��
��5��A2-ԭ�Ӻ�����x�����ӣ���������Ϊm����n g A2-�������ӵ����ʵ���Ϊ__________________��
���𰸡� 2OH-+SiO2==SiO32-+H2O SiO2+4HF=SiF4��+2H2O SiO32-+H2O+CO2=H2SiO3��+CO32- CO32- +2H+=H2O+CO2����SiO32-+2H+=H2SiO3�� ����� AC E 10��9 8��9 ![]() (m-x+2) mol
(m-x+2) mol
����������1������������������������Һ��Ӧ������Ժ�ǿ�Ĺ����ƣ���˲����ò���������Ӧ�����ӷ���ʽΪ2OH-+SiO2��SiO32-+H2O�����������������跴Ӧ����˲����Լ�ƿ����ʢ������ᣬ��Ӧ�Ļ�ѧ����ʽΪSiO2+4HF��SiF4��+2H2O����2��ʵ�����е�Na2SiO3��Һ���ڷ��ã�ƿ����ְ�ɫ���������������տ����еĶ�����̼���ɹ�����������γɳ��������ӷ���ʽ��SiO32-+H2O+CO2��H2SiO3��+CO32-����Һ�к���̼���κ����Σ�����ȡƿ�е��ϲ���Һ����ϡ���ᣬ�������ݲ������г������ɣ������ӷ���ʽ�ֱ�ΪCO32-+2H+��H2O+CO2����SiO32-+2H+��H2SiO3������Na2SiO3��Һ���ݹ���������ȼ�գ�����Na2SiO3����;�����������ԭ�ϡ���3�������Һ�м��������NaOH������ʱ����Ӧ���ɵİ����ݳ�����ͬʱ����Mg��OH��2��Fe��OH��2��Fe��OH��3������NaAlO2��Fe��OH��2�����ڿ����в��ȶ���Ѹ����������Fe��OH��3�����������м���������ᣬ��Mg��OH��2��Fe��OH��3��NaAlO2����������÷ֱ�����MgCl2��AlCl3��FeCl3������ٵ�������Ҫ�У�NH4+��Fe2+����ѡAC���������ӵ���������Fe3+����ѡE����4��H216O��D216O������������10�������������ͬ��H216O��D216O����������֮��ΪĦ������֮�ȵķ��ȣ���Ϊ20��18��10��9��H216O��D216O���������ֱ���8��10������������֮��Ϊ10��8��9��10��8��9����5��A2-ԭ�Ӻ�����x�����ӣ���������Ϊm������������m��x����������m��x+2�����n g A2-�������ӵ����ʵ���Ϊ![]() ��
��